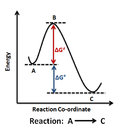
rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (Ea)...
19 KB (2,158 words) - 10:23, 26 September 2024
chemistry and biology, activation is the process whereby something is prepared or excited for a subsequent reaction. In chemistry, "activation" refers to the...
5 KB (566 words) - 02:03, 13 June 2024
G^{\ddagger }} is the Gibbs energy of activation, Δ S ‡ {\displaystyle \Delta S^{\ddagger }} is the entropy of activation, Δ H ‡ {\displaystyle \Delta...
21 KB (2,788 words) - 21:20, 24 April 2024
Activation energy asymptotics (AEA), also known as large activation energy asymptotics, is an asymptotic analysis used in the combustion field utilizing...
5 KB (825 words) - 09:12, 10 July 2024
an energy barrier known as the activation energy. The speed of a chemical reaction (at a given temperature T) is related to the activation energy E by...
59 KB (7,390 words) - 23:44, 26 September 2024
Chemical kinetics (section Free energy)
reactions, a steady state approximation can simplify the rate law. The activation energy for a reaction is experimentally determined through the Arrhenius...
24 KB (3,329 words) - 05:27, 10 July 2024
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol G {\displaystyle G} ) is a thermodynamic potential that can...
33 KB (4,546 words) - 15:39, 17 July 2024
Activation energy, which is defined as the amount of energy required to make the reaction start and carry on spontaneously. Higher activation energy implies...
66 KB (8,031 words) - 09:58, 12 July 2024
Catalysis (section Energy processing)
reactions. These pathways have lower activation energy. Consequently, more molecular collisions have the energy needed to reach the transition state....
46 KB (5,235 words) - 10:42, 26 September 2024
Marcus theory (redirect from Reorganization energy)
reaction path, but nevertheless one does observe an activation energy. The rate equation for activation-controlled reactions has the same exponential form...
40 KB (5,759 words) - 21:12, 17 April 2024
all catalysts, enzymes increase the reaction rate by lowering its activation energy. Some enzymes can make their conversion of substrate to product occur...
96 KB (9,819 words) - 12:03, 26 July 2024
Transition state theory (redirect from Activated complex theory)
activation) using experimental rate data. These so-called activation parameters give insight into the nature of a transition state, including energy content...
41 KB (5,817 words) - 07:25, 23 July 2024
starting material must have enough energy to cross over an energy barrier. This energy barrier is known as activation energy (∆G≠) and the rate of reaction...
29 KB (3,604 words) - 15:06, 13 February 2024
reaction. The activation energy is the minimum amount of energy to initiate a chemical reaction and form the activated complex. The energy serves as a threshold...
7 KB (913 words) - 01:06, 23 September 2024
kinetic energy required for a reaction to occur is called the activation energy and is denoted by Ea or ΔG‡. The transition state or activated complex...
26 KB (3,716 words) - 19:46, 19 September 2024
Evans–Polanyi–Semenov principle) observes that the difference in activation energy between two reactions of the same family is proportional to the difference...
3 KB (326 words) - 13:24, 31 July 2024
Desorption (section Phonon activated desorption)
This occurs when a molecule gains enough energy to overcome the activation barrier and the binding energy that keep it attached to the surface. Desorption...
20 KB (2,902 words) - 17:26, 25 September 2024
Polyvinyl nitrate (section Activation energy)
activation energy, R is the universal gas constant, T is absolute temperature, and C is a constant, dependent on the material. The activation energy is...
9 KB (1,348 words) - 06:17, 14 August 2023
enthalpy of activation ΔH‡ and the entropy of activation ΔS‡, based on the defining formula ΔG‡ = ΔH‡ − TΔS‡. In effect, the free energy of activation takes...
17 KB (2,392 words) - 03:06, 31 May 2024
Spin-forbidden reactions (section C-H activation)
are known as spin-forbidden reactions Such reactions show increased activation energy when compared to a similar reaction in which the spin states of the...
12 KB (1,328 words) - 19:41, 7 August 2024
double bond be broken, so that the activation energy is roughly 80 kJ/mol (20 kcal/mol). However, the activation energy can be lowered (and the isomerization...
12 KB (1,388 words) - 05:15, 27 April 2024
via a transition state. The process of getting to the top of the activation energy barrier to the transition state is endergonic. However, the reaction...
7 KB (938 words) - 07:14, 24 October 2023
less distortion energy (1.4 kcal/mol versus 4.6 kcal/mol) resulting in a lower activation energy despite smaller interaction energy. Decreasing transition...
44 KB (5,098 words) - 05:29, 19 February 2024
such as a flame or spark. This temperature is required to supply the activation energy needed for combustion. The temperature at which a chemical ignites...
8 KB (528 words) - 16:31, 30 August 2024
Overpotential (section Activation overpotential)
depends on the activation energy of the redox event. While ambiguous, "activation overpotential" often refers exclusively to the activation energy necessary...
9 KB (959 words) - 04:02, 26 April 2024
electrical device). There are also many other ways to bring sufficient activation energy including electricity, radiation, and pressure, all of which will...
13 KB (1,786 words) - 19:45, 10 September 2024
potential energy curves of a chemical reaction as a function of reaction coordinate (ζ), as portrayed in reaction coordinate diagrams. The activation strain...
13 KB (1,887 words) - 05:17, 22 April 2024
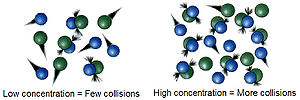
collisions. The successful collisions must have enough energy, also known as activation energy, at the moment of impact to break the pre-existing bonds...
22 KB (3,224 words) - 17:23, 31 July 2024
This process is achieved by lowering the activation energy of the reaction, so more substrates have enough energy to undergo reaction. Usually, an enzyme...
41 KB (4,859 words) - 19:37, 18 September 2024
Aquilanti–Mundim deformed Arrhenius model (section Apparent Reciprocal Activation Energy or Transitivity)
temperature. The meaning attached to the energy of activation E o {\displaystyle E_{o}} is as the minimum energy, which molecules need have to overcome...
39 KB (4,903 words) - 07:28, 23 July 2024