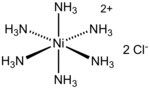
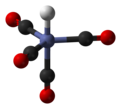
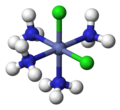
coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia (NH3) ligand. "Ammine" is spelled this way for historical...
16 KB (1,787 words) - 12:15, 19 July 2024
(disambiguation) Amino (disambiguation) Anime, Japanese animation Metal ammine complex This disambiguation page lists articles associated with the title...
419 bytes (87 words) - 16:31, 31 October 2022
Hexaamminenickel chloride (category Ammine complexes)
It is the chloride salt of the metal ammine complex [Ni(NH3)6]2+. The cation features six ammonia (called ammines in coordination chemistry) ligands attached...
4 KB (256 words) - 20:17, 6 August 2024
Hexaamminecobalt(III) chloride (category Ammine complexes)
of coordination chemistry, Alfred Werner. The cation itself is a metal ammine complex with six ammonia ligands attached to the cobalt(III) ion. [Co(NH3)6]3+...
8 KB (665 words) - 21:00, 6 August 2024
forming metal ammine complexes. For historical reasons, ammonia is named ammine in the nomenclature of coordination compounds. One notable ammine complex is...
139 KB (14,973 words) - 07:42, 11 November 2024
Chloropentammineplatinum chloride (redirect from Platinum(IV) ammine)
→ [PtCl(NH3)5]Cl3 + 2 KCl The title complex is one of several platinum ammine complexes. Hexaammineplatinum(IV) chloride Trichlorotriammineplatinum(IV)...
3 KB (158 words) - 20:15, 6 August 2024
dissolves in excess aqueous ammonia to form a colorless, water-soluble ammine complex. One major use is as an absorbent in surgical dressings. It is also...
4 KB (348 words) - 10:30, 27 August 2024
ammine groups. Its formula is [CuCl2(NH3)2]. The mineral is chemically pure. It was found in a guano deposit in Chile. At the same site other ammine-containing...
4 KB (272 words) - 05:40, 21 February 2023
of ammonium carbonate, ammonia, and oxygen to ultimately give copper(II) ammine complex carbonates, such as [Cu(NH3)4]CO3. After extraction from the residues...
10 KB (829 words) - 15:20, 14 September 2024
Metal amides (section Amido-ammine complexes)
coordinated amine oxidative addition of an amine Highly cationic metal ammine complexes such as [Pt(NH3)6]4+ spontaneously convert to the amido derivative:...
7 KB (773 words) - 10:45, 29 June 2024
example strychnine. Acid-base extraction Amine value Amine gas treating Ammine Biogenic amine Ligand isomerism Official naming rules for amines as determined...
36 KB (3,789 words) - 11:52, 8 November 2024
→ [LnMNO] + OH− The reaction is reversible in some cases. In some metal-ammine complexes, the ammonia ligand can be oxidized to nitrosyl: H2O + [Ru(terpy)(bipy)(NH3)]+...
17 KB (2,019 words) - 15:22, 2 September 2024
nitrogen triiodide (NI3), or more precisely, the slightly more stable ammine, NI3 · NH3. "Ammonium triiodide | H4I3N - PubChem". pubchem.ncbi.nlm.nih...
2 KB (66 words) - 02:14, 17 November 2023
isocyanurate mineral, with the formula Cu(C3N3O3H2)2(NH3)2. It is also an ammine-containing mineral, a feature shared with ammineite, chanabayaite and shilovite...
3 KB (194 words) - 16:16, 18 February 2023
members of such complexes are described in metal aquo complexes, metal ammine complexes, Examples: [Co(EDTA)]−, [Co(NH3)6]3+, [Fe(C2O4)3]3- Organometallic...
57 KB (5,553 words) - 04:29, 2 October 2024
often produced by salt metathesis reaction or by deprotonation of metal ammine complexes. Bergstrom, F. W. (1940). "Sodium Amide". Organic Syntheses. 20:...
4 KB (271 words) - 11:05, 22 October 2024
complexes with tiron. Beryllium has generally a rather poor affinity for ammine ligands. Ligands such as EDTA behave as dicarboxylic acids.[citation needed]...
98 KB (10,510 words) - 03:24, 1 November 2024
understand the difference between coordinated and ionic chloride in the cobalt ammine chlorides and to explain many of the previously inexplicable isomers. He...
35 KB (3,307 words) - 15:24, 6 November 2024
hydroxide oxidizes of ammonia in presence of oxygen, giving rise to copper ammine nitrites, such as Cu(NO2)2(NH3)n. Copper(II) hydroxide is mildly amphoteric...
14 KB (1,393 words) - 00:09, 21 October 2024
Silver bromide reacts readily with liquid ammonia to generate a variety of ammine complexes, like Ag(NH 3) 2Br and Ag(NH 3) 2Br− 2. In general: AgBr + m NH3...
18 KB (1,957 words) - 02:52, 31 October 2024
Hexaammineplatinum(IV) chloride (category Ammine complexes)
It is the chloride salt of the metal ammine complex [Pt(NH3)6]4+. The cation features six ammonia (called ammines in coordination chemistry) ligands attached...
3 KB (182 words) - 21:00, 6 August 2024
allergenicity being Cl > Br > I. Neutral compounds such as cis-platin and ammine and nitro complexes such as [Pt(NH3)4]Cl2, K2[Pt(NO2)4] and platinum nitrate...
3 KB (368 words) - 02:48, 13 January 2024
chloride, [Co(NH3)6]3+Cl−3. Here, (NH3)6 indicates that the ion contains six ammine groups (NH3) bonded to cobalt, and [ ] encloses the entire formula of the...
24 KB (3,205 words) - 14:13, 13 July 2024
variety of coordination complexes with ammonia and amines, which are called ammine complexes. Examples include [Co(NH3)6]3+, [Co(NH3)5Cl]2+ (chloropentamminecobalt(III))...
117 KB (12,028 words) - 02:36, 10 November 2024
Hexamminecobalt(III) chloride is a salt of a coordination complex wherein six ammonia ("ammine") ligands occupy the first coordination sphere of the ion Co3+....
7 KB (723 words) - 07:50, 6 May 2024
some of the islands given as Kanathara (Kavaratti), Argidion (Agatti), Ammine (Amini), and Monache (Minicoy). The islands later became part of a trade...
79 KB (6,438 words) - 01:28, 3 November 2024
commercial company is using a strontium chloride-based artificial solid called AdAmmine as a means to store ammonia at low pressure, mainly for use in NOx emission...
11 KB (923 words) - 20:03, 20 August 2024
Schweizer's reagent (category Ammine complexes)
Schweizer's reagent is a metal ammine complex with the formula Cu(NH3)4(H2O)2](OH)2. This deep-blue compound is used in purifying cellulose. This salt...
7 KB (734 words) - 10:47, 20 October 2024
Silver diammine fluoride (category Ammine complexes)
fluoride" (with one m); however, this is a misnomer, as SDF contains two ammine (NH3) groups, not amine (NH2) groups. Based on the current, best available...
33 KB (3,848 words) - 06:53, 19 May 2024
(1933). "Trimethyl Gallium, Trimethyl Gallium Etherate and Trimethyl Gallium Ammine". PNAS. 19 (3): 292–8. Bibcode:1933PNAS...19..292K. doi:10.1073/pnas.19...
15 KB (1,386 words) - 13:22, 12 July 2024