Beryllium oxalate is an inorganic compound, a salt of beryllium metal and oxalic acid with the chemical formula C 2BeO 4. It forms colorless crystals...
4 KB (293 words) - 17:38, 25 September 2023
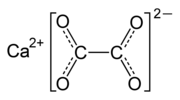
Calcium oxalate (in archaic terminology, oxalate of lime) is a calcium salt of oxalic acid with the chemical formula CaC2O4 or Ca(COO)2. It forms hydrates...
16 KB (1,485 words) - 06:02, 27 June 2024
Lithium oxalate is an organic compound with the chemical formula Li2C2O4. It is a salt of lithium metal and oxalic acid. It consists of lithium cations...
4 KB (257 words) - 04:37, 15 April 2024
Lanthanum oxalate is an inorganic compound, a salt of lanthanum metal and oxalic acid with the chemical formula La 2(C 2O 4) 3. Reaction of soluble lanthanum...
4 KB (287 words) - 15:21, 25 September 2023
Strontium oxalate is a compound with the chemical formula SrC2O4. Strontium oxalate can exist either in a hydrated form (SrC2O4·nH2O) or as the acidic...
3 KB (174 words) - 19:40, 12 May 2024
Alkaline earth metal (redirect from Beryllium family)
are six chemical elements in group 2 of the periodic table. They are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium...
71 KB (7,210 words) - 05:17, 10 August 2024
125 178 Beryllium oxalate trihydrate BeC2O4·3H2O 63.5 Beryllium perchlorate Be(ClO4)2 147 Beryllium selenate tetrahydrate BeSeO4·4H2O 49 Beryllium sulfate...
84 KB (193 words) - 10:24, 24 July 2024
Beryllium borohydride – Be[BH4]2 Beryllium bromide – BeBr2 Beryllium carbonate – BeCO3 Beryllium chloride – BeCl2 Beryllium fluoride – BeF2 Beryllium...
119 KB (8,734 words) - 09:23, 30 July 2024
cause most of the toxicity. The diagnosis may be suspected when calcium oxalate crystals are seen in the urine or when acidosis or an increased osmol gap...
61 KB (6,616 words) - 08:18, 15 May 2024
pressed with beryllium is also an efficient neutron source with the activity exceeding that of the standard americium-beryllium and radium-beryllium pairs....
42 KB (4,651 words) - 16:42, 9 August 2024
solubility of the most soluble hydrate is shown. Some compounds, such as nickel oxalate, will not precipitate immediately even though they are insoluble, requiring...
35 KB (1,083 words) - 11:43, 19 May 2024
[no] [no] Nabesite (IMA2000-024) 9.EA.65 [8] [9] [no] (IUPAC: disodium beryllium decaoxytetrasilicate tetrahydrate) Nabiasite (IMA1997-050) 8.BF.20 [10]...
39 KB (2,824 words) - 17:47, 9 February 2024
(IMA1973-055) 10.CA.40 [95] [96] [97] (IUPAC: uric acid) Uroxite (hydrous uranyl oxalate: IMA2018-100) 10.AB. [98] [no] [no] Ursilite (Y: 1957) 9.AK.35 [99] [100]...
37 KB (2,568 words) - 17:45, 9 February 2024
to X-ray imaging. When mixed with beryllium, radium acts as a neutron source. As of 2004[update], radium-beryllium neutron sources are still sometimes...
75 KB (8,164 words) - 02:32, 20 August 2024
metals; the lighter beryllium and magnesium, also in group 2 of the periodic table, are often included as well. Nevertheless, beryllium and magnesium differ...
47 KB (5,901 words) - 04:36, 20 August 2024
nitrate, perchlorate, and sulfate and is precipitated as a fluoride, oxalate, or hydroxide. Californium is the heaviest actinide to exhibit covalent...
44 KB (4,615 words) - 18:40, 29 June 2024
white solid. Yttrium forms a water-insoluble fluoride, hydroxide, and oxalate, but its bromide, chloride, iodide, nitrate and sulfate are all soluble...
58 KB (6,666 words) - 08:13, 6 August 2024
oxide of 241Am pressed with beryllium is an efficient neutron source. Here americium acts as the alpha source, and beryllium produces neutrons owing to...
76 KB (9,245 words) - 19:49, 16 August 2024
silver acetylide 7659–31–6 Ag2CO3 silver carbonate 534–16–7 Ag2C2O4 silver oxalate 533–51–7 Ag2Cl2 disilver dichloride 75763–82–5 Ag2CrO4 silver chromate...
139 KB (120 words) - 17:07, 15 July 2024
SiO2−3 Aluminium silicate AlSiO−4 Anions from organic acids Acetate CH3COO− ethanoate Formate HCOO− methanoate Oxalate C2O2−4 ethanedioate Cyanide CN−...
30 KB (3,020 words) - 20:58, 7 May 2024
accident in the factory number 20 in the collection oxalate decantate after filtering sediment oxalate enriched uranium. Six people received doses of 300...
120 KB (11,802 words) - 05:47, 14 August 2024
hydrofluoric acid, and phosphoric acid, giving solid precipitates of barium oxalate, fluoride, and phosphate, respectively. Barium bromide can be prepared...
8 KB (426 words) - 04:11, 8 August 2024
control the sulfate concentration in the feed brine for electrolysis. Oxalate effects a similar reaction: BaCl2 + Na2C2O4 → 2 NaCl + BaC2O4 When it is...
13 KB (1,067 words) - 19:45, 12 May 2024
silver amide, and silver fulminate, as well as silver acetylide, silver oxalate, and silver(II) oxide. They can explode on heating, force, drying, illumination...
94 KB (11,248 words) - 11:22, 15 August 2024
acetylide 7659-31-6 Ag2CO3 silver(I) carbonate 534-16-7 Ag2C2O4 silver oxalate 533-51-7 Ag2Cl2 silver(II) dichloride 75763-82-5 Ag2CrO4 silver chromate...
182 KB (107 words) - 15:39, 24 July 2024
parlance of the day), which he called yttria. Anders Gustav Ekeberg isolated beryllium from the gadolinite but failed to recognize other elements in the ore...
157 KB (16,855 words) - 14:52, 19 August 2024
photographic processes. The dihydrate of iron(II) oxalate has a polymeric structure with co-planar oxalate ions bridging between iron centres with the water...
150 KB (17,052 words) - 13:09, 16 August 2024
the solution is treated with ammonium oxalate to convert rare earths to their insoluble oxalates. The oxalates are converted to oxides by annealing. The...
48 KB (6,041 words) - 11:33, 13 August 2024
Pm2(SO4)3·8H2O is 2.86 g/cm3. The oxalate, Pm2(C2O4)3·10H2O, has the lowest solubility of all lanthanide oxalates. Unlike the nitrate, the oxide is similar...
40 KB (4,785 words) - 08:03, 13 August 2024
solution. The alkaline earth metals were precipitated either as sulfates or oxalates, leaving the alkali metal in the solution. After conversion to the nitrates...
93 KB (10,047 words) - 15:33, 13 August 2024