A boronic acid is an organic compound related to boric acid (B(OH)3) in which one of the three hydroxyl groups (−OH) is replaced by an alkyl or aryl group...
27 KB (2,662 words) - 03:08, 9 November 2023
groups attached to boron. Phenylboronic acid is a white powder and is commonly used in organic synthesis. Boronic acids are mild Lewis acids which are generally...
9 KB (882 words) - 15:14, 22 May 2024
Protodeboronation (category Boron compounds)
metal-catalysed coupling reactions that utilise boronic acids (see Suzuki reaction). For a given boronic acid, the propensity to undergo protodeboronation...
11 KB (1,438 words) - 11:45, 14 February 2023
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula B(OH)3. It may also be called hydrogen orthoborate...
47 KB (5,000 words) - 13:49, 28 August 2024
modern climate. Allotropes of boron Boron deficiency Boron oxide Boron nitride Boron neutron capture therapy Boronic acid Hydroboration-oxidation reaction...
127 KB (13,477 words) - 16:02, 23 September 2024
reactions include availability of common boronic acids, mild reaction conditions, and its less toxic nature. Boronic acids are less toxic and safer for the environment...
34 KB (3,845 words) - 17:47, 26 September 2024
Petasis reaction (category Chemical synthesis of amino acids)
multi-component reaction of an amine, a carbonyl, and a vinyl- or aryl-boronic acid to form substituted amines. Reported in 1993 by Nicos Petasis as a practical...
29 KB (3,232 words) - 12:16, 21 July 2024
Dynamic covalent chemistry (section Boronic acid)
natural systems. Boronic acid self-condensation or condensation with diols is a well-documented dynamic covalent reaction. The boronic acid condensation has...
18 KB (1,991 words) - 05:20, 1 December 2023
Organoboron chemistry (redirect from Carbon-boron bond)
BRn(OR)3-n are called borinic esters (n = 2), boronic esters (n = 1), and borates (n = 0). Boronic acids are key to the Suzuki reaction. Trimethyl borate...
30 KB (2,992 words) - 19:24, 18 August 2024
Functional group (section Groups containing boron)
Compounds containing boron exhibit unique chemistry due to their having partially filled octets and therefore acting as Lewis acids. note 1 Fluorine is...
31 KB (1,230 words) - 16:08, 30 September 2024
Chan–Evans–Lam coupling is a cross-coupling reaction between an aryl boronic acid and an alcohol or an amine to form the corresponding secondary aryl amines...
5 KB (622 words) - 17:13, 26 September 2024
groups to make boronic and borinic esters. Purification of the mixtures that result from the reactions is required, as often boronic esters will also...
18 KB (1,880 words) - 20:34, 8 August 2024
inhibitor than would be necessary in an unhydrolyzable inhibitor. Different boronic acid derivatives have the potential to be tailored to the many different isoforms...
16 KB (1,696 words) - 15:55, 25 July 2024
shown to conveniently detect peptides incorporating the boronic acid moiety by MALDI. Gentisic acid - Compound Summary, PubChem. Haynes, p. 5.91 Haynes,...
5 KB (415 words) - 18:01, 4 January 2024
Borylation (category Boron)
molecules. Boronic acids, and boronic esters are common boryl groups incorporated into organic molecules through borylation reactions. Boronic acids are trivalent...
35 KB (3,976 words) - 11:26, 22 April 2024
Ester (redirect from Carboxylic acid ester)
oxoacids (e.g. esters of acetic acid, carbonic acid, sulfuric acid, phosphoric acid, nitric acid, xanthic acid), but also from acids that do not contain oxygen...
45 KB (4,782 words) - 16:16, 18 September 2024
Carbonyl group (redirect from Carboxylic Acid Derivatives)
classes of organic compounds (such as aldehydes, ketones and carboxylic acids), as part of many larger functional groups. A compound containing a carbonyl...
9 KB (875 words) - 20:45, 15 July 2024
organic reaction forming a new carbon–carbon bond from a thioester and a boronic acid using a metal catalyst. It is a cross-coupling reaction. This reaction...
12 KB (1,399 words) - 02:46, 6 March 2023
methods are not or only conditionally usable. In addition to the organic boronic acid derivatives, which often bind highly specifically to the 1,2-diol groups...
121 KB (12,789 words) - 20:47, 6 September 2024
alternatives to boronic acids (RB(OH)2), boronate esters (RB(OR′)2), and organoboranes (R3B), particularly for Suzuki-Miyaura coupling. Boronic acids RB(OH)2...
5 KB (347 words) - 19:08, 1 June 2024
carboxylic acid is an organic acid that contains a carboxyl group (−C(=O)−OH) attached to an R-group. The general formula of a carboxylic acid is often...
26 KB (2,619 words) - 01:49, 26 August 2024
Nevalainen T (November 2008). "Discovery of boronic acids as novel and potent inhibitors of fatty acid amide hydrolase". Journal of Medicinal Chemistry...
2 KB (154 words) - 11:39, 23 January 2023
forms white fumes in moist air. It is a useful Lewis acid and a versatile building block for other boron compounds. The geometry of a molecule of BF3 is trigonal...
19 KB (1,848 words) - 16:23, 29 August 2024
protected aryl boronic ester which gives after acidic work-up the target product in 78% yield. The same reactants are forming with the aryl boronic ester at...
9 KB (908 words) - 12:26, 9 July 2023
Although ethers resist hydrolysis, they are cleaved by hydrobromic acid and hydroiodic acid. Hydrogen chloride cleaves ethers only slowly. Methyl ethers typically...
19 KB (1,835 words) - 11:18, 2 September 2024
Tetrahydroxydiboron (category Boron compounds)
Tetrahydroxydiboron is a chemical reagent which can be used to prepare boronic acids. The reaction of boron trichloride with alcohols was reported in 1931, and was used...
5 KB (327 words) - 21:16, 18 September 2024
peroxides are: Peroxy acids, the peroxy derivatives of many familiar acids, examples being peroxymonosulfuric acid and peracetic acid, and their salts, one...
5 KB (540 words) - 01:42, 26 September 2024
an antihistamine used to treat allergies. Phenylalanine, a common amino acid. Biphenyl, consisting of two phenyl groups. The two rings tend not to be...
9 KB (1,044 words) - 16:54, 8 May 2024
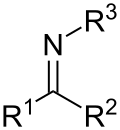
Imine (section Acid-base reactions)
carbon acids with nitroso compounds. The rearrangement of trityl N-haloamines in the Stieglitz rearrangement. By reaction of alkenes with hydrazoic acid in...
23 KB (2,633 words) - 01:56, 2 September 2024
published the Suzuki reaction, the organic reaction of an aryl- or vinyl-boronic acid with an aryl- or vinyl-halide catalyzed by a palladium(0) complex, in...
11 KB (948 words) - 02:40, 14 September 2024