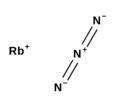
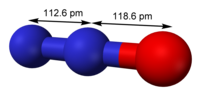
Caesium azide or cesium azide is an inorganic compound of caesium and nitrogen. It is a salt of azide with the formula CsN3. CsN3 adopts the same structure...
3 KB (276 words) - 20:50, 12 May 2024
fused caesium cyanide (CsCN). Exceptionally pure and gas-free caesium can be produced by 390 °C (734 °F) thermal decomposition of caesium azide CsN 3...
93 KB (10,047 words) - 15:33, 13 August 2024
Because metallic caesium is too reactive to handle, it is normally offered as caesium azide (CsN3). Caesium hydroxide is formed when caesium interacts aggressively...
214 KB (23,508 words) - 00:37, 13 August 2024
Ammonium azide is the chemical compound with the formula [NH4]N3, being the salt of ammonia and hydrazoic acid. Like other inorganic azides, this colourless...
5 KB (244 words) - 14:03, 3 August 2024
rubidium azide has the same structure as potassium hydrogen fluoride; a distorted caesium chloride structure. At 315 °C and 1 atm, rubidium azide will transition...
8 KB (705 words) - 23:28, 19 March 2024
0.04642 Caesium acetate CsC2H3O2 1010 1345.5 Caesium azide CsN3 307 Caesium bromate CsBrO3 2.10 3.66 4.53 5.3 Caesium bromide CsBr 108 Caesium chlorate...
84 KB (193 words) - 10:24, 24 July 2024
spellings may differ, such as aluminum/aluminium, sulfur/sulphur, and caesium/cesium. Contents A B C Ca–Cu D E F G H I K L M N O P R S T U V W X Y Z...
182 KB (107 words) - 15:39, 24 July 2024
names of many nitrogen compounds, such as hydrazine and compounds of the azide ion. Finally, it led to the name "pnictogens" for the group headed by nitrogen...
105 KB (12,223 words) - 08:00, 14 August 2024
CsNO2 caesium nitrite 13454–83–6 CsNO3 caesium nitrate 7789–18–6 CsN3 caesium azide 22750–57–8 CsOH caesium hydroxide 21351–79–1 CsO2 caesium superoxide...
139 KB (120 words) - 17:07, 15 July 2024
aldehydes. This protocol takes advantage of a stable sulfonyl azide, rather than tosyl azide, for the in situ generation of the Ohira−Bestmann reagent. Another...
6 KB (529 words) - 03:16, 22 March 2024
CdTe Caesium bicarbonate – CsHCO3 Caesium carbonate – Cs2CO3 Caesium chloride – CsCl Caesium chromate – Cs2CrO4 Caesium fluoride – CsF Caesium hydride...
119 KB (8,734 words) - 09:23, 30 July 2024
unstable and spontaneously explode. For example, the rubidium, potassium and caesium salts are so unstable that they explode while crystallizing. Azidotetrazolates...
6 KB (512 words) - 10:53, 24 June 2024
reprotected as BOC 6 and the azide group converted to the amide 7 by reductive acylation with thioacetic acid and 2,6-lutidine. Caesium carbonate accomplishes...
20 KB (2,309 words) - 12:58, 25 October 2023
which is folded into a long, narrow trough, filled with a mixture of barium azide and powdered glass, and then folded into the closed ring shape. The getter...
13 KB (1,688 words) - 23:41, 27 December 2023
the crude product. A second method involves heating a mixture of sodium azide and sodium nitrate: 5 NaN3 + NaNO3 → 3 Na2O + 8 N2 Burning sodium in air...
6 KB (542 words) - 07:51, 30 April 2024
electrolysis. Cobalt(II) azide (Co(N3)2) is another binary compound of cobalt and nitrogen that can explode when heated. Cobalt(II) and azide can form Co(N 3)2−...
16 KB (1,837 words) - 16:35, 13 November 2023
ferrous Fe2+, cobalt Co2+ caesium Cs+ and lead Pb2+ cations. Borderline bases are: aniline, pyridine, nitrogen N2 and the azide, chloride, bromide, nitrate...
19 KB (2,114 words) - 13:17, 17 August 2024
identified in 1952 as the thermal decomposition product of the fluorine azide (FN3). It has the structure F−N=N−F and exists in both cis and trans isomers...
7 KB (574 words) - 04:35, 14 February 2024
reaction is the route adopted by the commercial chemical industry to produce azide salts, which are used as detonators. The gas was first synthesised in 1772...
96 KB (10,049 words) - 23:21, 1 August 2024
hydrosilanes include amides, and α,β-unsaturated esters enamines, imines, and azides. Trifluoroacetic acid, often used in these reductions, is a strong, corrosive...
13 KB (1,452 words) - 16:57, 15 August 2024
dangerously explosive silver compounds are silver azide, AgN3, formed by reaction of silver nitrate with sodium azide, and silver acetylide, Ag2C2, formed when...
94 KB (11,248 words) - 11:22, 15 August 2024
hemoglobin and certain cytochromes in a manner analogous to cyanide and azide (see below, under precautions). The two principal sulfur oxides are obtained...
99 KB (11,030 words) - 21:33, 18 August 2024
such as cyanogen bromide (BrCN), bromine thiocyanate (BrSCN), and bromine azide (BrN3). The pale-brown bromine monofluoride (BrF) is unstable at room temperature...
67 KB (7,693 words) - 20:33, 10 August 2024
in ionized form it is commonly found with one gained electron, as Cl−. Caesium has the lowest measured ionization energy of all the elements and helium...
30 KB (3,020 words) - 20:58, 7 May 2024
higher than those of the heavier alkali metals potassium, rubidium, and caesium, following periodic trends down the group. These properties change dramatically...
70 KB (8,195 words) - 18:55, 18 August 2024
principle by a consideration of ionic bonding. In the gas phase, molecular caesium fluoride has a polar covalent bond. The large difference in electronegativity...
8 KB (1,136 words) - 23:47, 13 July 2024
known, such as cyanogen iodide (ICN), iodine thiocyanate (ISCN), and iodine azide (IN3). Iodine monofluoride (IF) is unstable at room temperature and disproportionates...
106 KB (11,790 words) - 10:37, 13 August 2024
that same year, E. Eoler isolated radium by thermal decomposition of its azide, Ra(N3)2. Radium metal was first industrially produced at the beginning...
75 KB (8,164 words) - 02:32, 20 August 2024
chlorine thiocyanate (ClSCN, unlike its oxygen counterpart), and chlorine azide (ClN3). Chlorine monofluoride (ClF) is extremely thermally stable, and is...
117 KB (13,028 words) - 12:00, 13 August 2024
is a high-melting-point compound which is readily hydrolyzed. Beryllium azide, BeN6 is known and beryllium phosphide, Be3P2 has a similar structure to...
95 KB (10,200 words) - 00:33, 18 August 2024