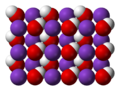
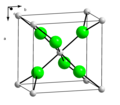
quicklime (calcium oxide) is mixed with water. Annually, approximately 125 million tons of calcium hydroxide are produced worldwide. Calcium hydroxide has many...
22 KB (2,126 words) - 09:10, 9 November 2024
broadly used term lime connotes calcium-containing inorganic compounds, in which carbonates, oxides, and hydroxides of calcium, silicon, magnesium, aluminium...
23 KB (2,231 words) - 17:52, 21 October 2024
Pulp capping (section Calcium hydroxide cement)
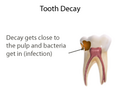
dentist will place a small amount of a sedative dressing, such as calcium hydroxide or mineral trioxide aggregate (MTA). These materials protect the pulp...
29 KB (3,539 words) - 17:21, 21 August 2024
the hydroxides of the heavier alkaline earths: calcium hydroxide, strontium hydroxide, and barium hydroxide. A solution or suspension of calcium hydroxide...
41 KB (4,891 words) - 13:03, 26 August 2024
Calcium hydroxychloride or calcium chloride hydroxide is an inorganic compound with the chemical formula Ca(OH)Cl. It consists of calcium cations (Ca2+)...
4 KB (372 words) - 13:56, 11 May 2024
Calcium hydrosulfide is the chemical compound with the formula Ca(HS) 2 or CaH 2S 2. It is formed from the reaction of calcium hydroxide or calcium carbonate...
2 KB (87 words) - 18:11, 8 August 2024
and tamales. Nixtamal is similar, but uses calcium hydroxide instead of sodium hydroxide. Sodium hydroxide is frequently used as an industrial cleaning...
54 KB (5,913 words) - 03:13, 29 October 2024
of calcium hydroxide (slaked lime). The salt metathesis reaction results in precipitation of solid calcium carbonate, leaving potassium hydroxide in solution:...
21 KB (2,063 words) - 06:28, 7 October 2024
This page provides supplementary chemical data on calcium hydroxide. The handling of this chemical may incur notable safety precautions. It is highly...
6 KB (191 words) - 15:03, 11 April 2023
produce calcium hydroxide and hydrogen gas. It also reacts with the oxygen and nitrogen in air to form a mixture of calcium oxide and calcium nitride...
47 KB (5,905 words) - 03:21, 30 October 2024
Lime (material) (category Calcium minerals)
an inorganic material composed primarily of calcium oxides and hydroxides. It is also the name for calcium oxide which occurs as a product of coal-seam...
19 KB (2,351 words) - 19:28, 30 October 2024
double salt of calcium nitrite/calcium hydroxide; and in the presence of water, decomposing double salt to form a solution of calcium nitrite and insolubilize...
8 KB (840 words) - 08:17, 8 November 2023
pregnancy and breastfeeding. Calcium gluconate is made by mixing gluconic acid with calcium carbonate or calcium hydroxide. Calcium gluconate came into medical...
19 KB (1,605 words) - 02:28, 29 September 2024
ammonium nitrate, and calcium hydroxide: 2 NH4NO3 + Ca(OH)2 → Ca(NO3)2 + 2 NH4OH Like related alkaline earth metal nitrates, calcium nitrate decomposes upon...
12 KB (1,038 words) - 21:34, 13 August 2024
Base (chemistry) (section Alkalinity of non-hydroxides)
Barium hydroxide, magnesium hydroxide, calcium hydroxide, zinc hydroxide, iron(II) hydroxide, tin(II) hydroxide, lead(II) hydroxide, copper(II) hydroxide, etc...
26 KB (3,041 words) - 06:18, 8 August 2024
water. It can be created by neutralising hydrochloric acid with calcium hydroxide. Calcium chloride is commonly encountered as a hydrated solid with generic...
36 KB (2,912 words) - 02:56, 25 October 2024
reacting calcium hydroxide with sodium bromate or calcium sulfate with barium bromate. Above 180 °C, calcium bromate decomposes to form calcium bromide...
4 KB (142 words) - 20:20, 25 September 2023
gaseous hydrogen to form methane and water vapor plus solid calcium oxide or calcium hydroxide depending on temperature and product gas composition. Various...
79 KB (7,544 words) - 06:17, 8 November 2024
Concrete degradation (section Calcium leaching)
diffuse into concrete from its external surface, they react with calcium hydroxide (portlandite, Ca(OH)2) and the pH of the concrete pore water progressively...
62 KB (7,758 words) - 17:53, 6 November 2024
suspension of calcium hydroxide in water, producing calcium hypochlorite, which disproportionates when heated with excess chlorine to give calcium chlorate...
5 KB (402 words) - 00:32, 21 May 2024
Soda lime (category Hydroxides)
Soda lime, a mixture of sodium hydroxide (NaOH) and calcium oxide (CaO), is used in granular form within recirculating breathing environments like general...
12 KB (1,310 words) - 16:00, 4 October 2024
Pulpotomy (section Calcium hydroxide)
vital pulp such as Buckley's Solution of formocresol, ferric sulfate, calcium hydroxide or mineral trioxide aggregate (MTA). MTA is a more recent material...
25 KB (3,112 words) - 06:05, 31 August 2024
Magnesium hydroxide is an inorganic compound with the chemical formula Mg(OH)2. It occurs in nature as the mineral brucite. It is a white solid with low...
21 KB (2,031 words) - 11:40, 11 September 2024
Lime (material), inorganic materials containing calcium, usually calcium oxide or calcium hydroxide Lime (color), a color between yellow and green Lime...
3 KB (407 words) - 15:33, 29 November 2023
this substance with calcium hydroxide (slaked lime), a far more strongly basic substance known as caustic potash (potassium hydroxide) was produced. Caustic...
7 KB (794 words) - 09:34, 16 October 2024
Barium hydroxide is a chemical compound with the chemical formula Ba(OH)2. The monohydrate (x = 1), known as baryta or baryta-water, is one of the principal...
11 KB (927 words) - 01:27, 6 October 2024
and calcium hydroxide and hydrated calcium silicate in the concrete is known as neutralisation. The similar reaction in which calcium hydroxide from...
4 KB (445 words) - 01:23, 21 January 2024
lithium oxide. Lithium hydroxide is often produced industrially from lithium carbonate in a metathesis reaction with calcium hydroxide: Li2CO3 + Ca(OH)2 →...
14 KB (1,096 words) - 12:21, 25 August 2024
chemical reaction between the two substances, such as the reaction of calcium hydroxide with hydrochloric acid; even though one might say, informally, that...
50 KB (6,544 words) - 18:04, 30 September 2024
Kidney stone disease (redirect from Calcium stone)
be around 10 mm (0.4 in) of stone radius per month. Calcium hydroxide decreases urinary calcium when combined with food rich in oxalic acid such as green...
132 KB (13,797 words) - 23:46, 18 October 2024