Cerium(IV) hydroxide, also known as ceric hydroxide, is an inorganic compound with the chemical formula Ce(OH)4. It is a yellowish powder that is insoluble...
3 KB (273 words) - 19:22, 9 June 2024
Cerium hydroxide may refer to: Cerium(III) hydroxide, Ce(OH)3, cerium trihydroxide Cerium(IV) hydroxide, Ce(OH)4, cerium tetrahydroxide This set index...
490 bytes (55 words) - 14:35, 25 February 2021
Cerium(III) hydroxide is a hydroxide of the rare-earth metal cerium. It is a pale white powder with the chemical formula Ce(OH)3. Cerium(III) hydroxide...
2 KB (87 words) - 09:07, 26 November 2024
Thorium(IV) hydroxide is an inorganic compound with a chemical formula Th(OH)4. Thorium(IV) hydroxide can be produced by reacting sodium hydroxide and soluble...
2 KB (142 words) - 14:17, 25 September 2024
electropositive, cerium reacts with water. The reaction is slow with cold water but speeds up with increasing temperature, producing cerium(III) hydroxide and hydrogen...
52 KB (6,137 words) - 20:08, 14 December 2024
elements resist oxidation. Cerium(IV) oxide is formed by the calcination of cerium oxalate or cerium hydroxide. Cerium also forms cerium(III) oxide, Ce 2O 3...
24 KB (2,398 words) - 16:05, 19 December 2024
by reacting cerium(IV) hydroxide with hot selenic acid, and the tetrahydrate can be crystallized from the solution. Cerium(IV) selenate has a space group...
3 KB (239 words) - 09:27, 26 November 2024
Ce2S3 Cerium(IV) hydroxide – Ce(OH)4 Cerium(IV) nitrate – Ce(NO3)4 Cerium(IV) oxide – CeO2 Cerium(IV) sulfate – Ce(SO4)2 Cerium(III,IV) oxide – Ce3O4...
119 KB (8,721 words) - 20:49, 6 December 2024
Cerium nitrate refers to a family of nitrates of cerium in the +3 or +4 oxidation state. Often these compounds contain water, hydroxide, or hydronium...
25 KB (2,645 words) - 07:47, 26 November 2024
and G H I L M N and O P R S T U, V, and X Y Z References External links "Cerium (III) Acetate" (PDF). Retrieved 2019-09-28. "Magnesium Sulfite" (PDF). srdata...
84 KB (196 words) - 10:05, 30 October 2024
Lanthanide (section Oxides and hydroxides)
carbonates, hydroxides and hydroxycarbonates. They dissolve in acids to form salts. Cerium forms a stoichiometric dioxide, CeO2, where cerium has an oxidation...
107 KB (10,324 words) - 12:13, 27 November 2024
called didymium from a residue he called "lanthana", in turn separated from cerium salts. In 1885, the Austrian chemist Carl Auer von Welsbach separated didymium...
37 KB (4,921 words) - 02:43, 17 December 2024
Miltz (2003). "Thermal decomposition of the rare earth sulfates of cerium(III), cerium(IV), lanthanum(III) and samarium(III)". Applied Surface Science. 214...
6 KB (289 words) - 14:51, 19 December 2024
Gadolinium-doped ceria (category Cerium(IV) compounds)
as gadolinia-doped ceria, gadolinium-doped cerium oxide (GCO), cerium-gadolinium oxide (CGO), or cerium(IV) oxide, gadolinium-doped, formula Gd:CeO2)...
5 KB (613 words) - 16:35, 4 January 2024
which forms the dihydrate, TcO2·2H2O, which is also known as technetium(IV) hydroxide. It is a radioactive black solid which slowly oxidizes in air. Technetium...
9 KB (715 words) - 15:51, 19 December 2023
(PB28): cobalt(II) aluminate. Cerulean blue (PB35): cobalt(II) stannate. Cerium uranium blue Copper pigments Egyptian blue: a synthetic pigment of calcium...
9 KB (909 words) - 15:22, 30 November 2024
Zirconium selenate (redirect from Zirconium(IV) selenate)
saturated aqueous solution of zirconium oxychloride octahydrate (or zirconium hydroxide). The tetrahydrate belongs to the orthorhombic crystal system and is isostructural...
3 KB (226 words) - 15:46, 26 December 2023
Hydrolysis constant (section Cerium(III))
often applies to cations forming soluble hydroxide or oxide complexes with, in some cases, the formation of hydroxide and oxide precipitates. The hydrolysis...
123 KB (5,143 words) - 22:33, 21 November 2024
occurs in the +4 oxidation state, together with uranium(IV), zirconium(IV), hafnium(IV), and cerium(IV), and also with scandium, yttrium, and the trivalent...
137 KB (16,056 words) - 02:10, 23 December 2024
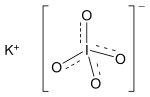
makes it useful for the determination of potassium[citation needed] and cerium. It is slightly soluble in water (one of the less soluble of potassium salts...
4 KB (326 words) - 15:56, 29 February 2024
periodate solution (until Ph. Eur. 3, Appendix 2001 also for iodine and cerium(IV) sulfate solutions, since Ph. Eur. 4, 2002 standardised by sodium thiosulfate)...
4 KB (402 words) - 03:17, 22 May 2024
Cerocene (category Cerium(III) compounds)
to produce cerium(III) hydroxide and cyclopentadiene. There are literature reports on the synthesis and properties of the tetravalent cerium compound Ce(C5H5)4...
4 KB (319 words) - 21:24, 6 January 2024
calcium and thorium. Of commercial interest are related ketonizations using cerium(IV) oxide and manganese dioxide on alumina as the catalysts. The synthesis...
9 KB (864 words) - 13:27, 4 December 2024
Astrocyanite-(Ce) (hydrated copper cerium neodymium lanthanum praseodymium samarium calcium yttrium uranyl carbonate hydroxide) Bayleyite (hydrated magnesium...
6 KB (508 words) - 22:29, 24 September 2022
Thorium compounds (section Oxides and hydroxides)
(ThBr4, white, m.p. 679 °C) can be produced either by reacting thorium(IV) hydroxide with hydrobromic acid (which has the disadvantage of often resulting...
35 KB (4,183 words) - 00:20, 24 November 2024
This process is also used to separate the lanthanides, such as lanthanum, cerium, neodymium, praseodymium, europium, and ytterbium, from each other. The...
20 KB (2,203 words) - 05:28, 21 October 2024
Lanthanide compounds (section Hydroxides)
tetrahalides, only the fluorides of cerium, praseodymium and terbium are well characterised. Neodymium(IV) fluoride and dysprosium(IV) fluoride are also known under...
43 KB (3,229 words) - 04:38, 4 June 2024
Praseodymium(III) hydroxide is an inorganic compound with a chemical formula Pr(OH)3. The reaction between ammonia water and praseodymium(III) nitrate...
2 KB (136 words) - 16:14, 4 January 2024
Neptunium (section Hydroxides)
other Np(IV) hydroxide, Np(OH)− 5, does not have a significant presence. Because the Np(V) ion NpO+ 2 is very stable, it can only form a hydroxide in high...
114 KB (13,744 words) - 07:49, 4 December 2024