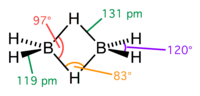
Diborane(6), commonly known as diborane, is the chemical compound with the formula B2H6. It is a highly toxic, colorless, and pyrophoric gas with a repulsively...
27 KB (2,596 words) - 21:25, 17 September 2024
There are other forms of diborane with different numbers of hydrogen atoms, including diborane(4) and diborane(6). Diborane(2) is a highly reactive molecule...
12 KB (1,222 words) - 12:07, 30 September 2024
Diborane(4) is a transient inorganic compound with the chemical formula B 2H 4. Stable derivatives are known. Diborane(4) has been produced by abstraction...
4 KB (299 words) - 04:06, 27 October 2023
chemical species, it reacts with itself to form diborane. Thus, it is an intermediate in the preparation of diborane according to the reaction: BX3 +BH4− → HBX3−...
13 KB (1,243 words) - 14:32, 17 August 2024
structure and bonding. First, new synthetic techniques were required to handle diborane and many of its derivatives, which are both pyrophoric and volatile. Alfred...
5 KB (561 words) - 03:08, 21 August 2024
molecules and ions are described as dimers, even when the monomer is elusive. Diborane (B2H6) is an dimer of borane, which is elusive and rarely observed. Almost...
13 KB (1,298 words) - 23:06, 7 October 2024
electrons in bonding. This type of bonding occurs in boron hydrides such as diborane (B2H6), which are often described as electron deficient because there are...
28 KB (3,673 words) - 00:25, 28 September 2024
reactions of diborane, B2H6. Hermann Irving Schlesinger's laboratory at the University of Chicago was one of two laboratories that prepared diborane. It was...
15 KB (1,386 words) - 05:55, 9 June 2024
and will burn readily (e.g., gasoline, acetylene, propane, hydrogen gas, diborane). Includes pyrophoric substances. Flash point below room temperature at...
14 KB (680 words) - 15:53, 1 October 2024
hydrogen. Unlike diborane, It is quite stable at room temperature if stored properly. It is much more stable in presence of water than diborane. Pentaborane...
11 KB (1,148 words) - 17:48, 25 September 2024
Example molecules with 3c–2e bonds are the trihydrogen cation (H+ 3) and diborane (B 2H 6). In these two structures, the three atoms in each 3c-2e bond form...
5 KB (688 words) - 12:00, 26 March 2023
Lifting gas (section Diborane)
far more expensive, rendering use as a lifting gas highly impractical. Diborane is slightly lighter than molecular nitrogen with a molecular mass of 27...
20 KB (2,644 words) - 06:11, 28 August 2024
hydrogen fuel, but is otherwise primarily of academic interest. Reaction of diborane with ammonia mainly gives the diammoniate salt [H2B(NH3)2]+[BH4]−...
13 KB (1,032 words) - 07:30, 6 September 2024
NaH in mineral oil is often directly used, such as in the production of diborane. NaH is a base of wide scope and utility in organic chemistry. As a superbase...
14 KB (1,339 words) - 02:55, 29 March 2024
solvents. Although usually purchased, BMS can be prepared by absorbing diborane into dimethyl sulfide: B2H6 + 2 S(CH3)2 → 2 BH3·S(CH3)2 It can be purified...
7 KB (591 words) - 22:09, 24 April 2024
reactions: mercuric acetate, water Hydroboration-oxidation reactions: diborane the Prins reaction: formaldehyde, water March, Jerry; (1985). Advanced...
2 KB (243 words) - 11:21, 17 April 2024
F− ↔ F− H−F] Another example is the three-center two-electron bond in diborane (B2H6). Maximum valences for the elements are based on the data from list...
40 KB (2,914 words) - 06:38, 15 August 2024
in fuel cells sodium hydride: a powerful base used in organic chemistry diborane: reducing agent, rocket fuel, semiconductor dopant, catalyst, used in organic...
21 KB (2,283 words) - 16:04, 30 September 2024
stretching vibration is A1g + Eg + T1u . Of these, only T1u is IR active. B2H6 (diborane) has D2h molecular symmetry. The terminal B-H stretching vibrations which...
47 KB (4,053 words) - 15:14, 9 October 2024
compound with the formula [(CH3)BH2]2. Structurally, it is related to diborane, but with methyl groups replacing terminal hydrides on each boron. It is...
17 KB (1,620 words) - 07:59, 17 October 2022
peroxide, but not truly pyrophoric. Nonmetal hydrides (arsine, phosphine, diborane, germane, silane) Metal carbonyls (dicobalt octacarbonyl, nickel carbonyl)...
9 KB (813 words) - 18:40, 29 June 2024
dimer Ga 2H 6 (digallane) is formed as a gas. Its structure is similar to diborane, having two hydrogen atoms bridging the two gallium centers,: 1031 unlike...
74 KB (8,761 words) - 07:52, 20 September 2024
Herbert C. Brown. Much of the original work on hydroboration employed diborane as a source of BH3. Usually however, borane dimethylsulfide complex BH3S(CH3)2...
13 KB (1,481 words) - 05:48, 22 August 2024
alkyldiboranes, consisting of a methyl group substituted for a hydrogen in diborane. As with other boranes it exists in the form of a dimer with a twin hydrogen...
21 KB (2,131 words) - 10:44, 3 January 2024
by adding phosphine, arsine, or diborane. Adding phosphine or arsine results in slower deposition, while adding diborane increases the deposition rate....
39 KB (4,395 words) - 15:05, 7 October 2024
atmosphere in fumigation as a flux for soldering magnesium to prepare diborane Boron trifluoride was discovered in 1808 by Joseph Louis Gay-Lussac and...
19 KB (1,848 words) - 16:23, 29 August 2024
macrobicyclic diamide. This di(tertiary)amide is reduced to the diamine by diborane. [2.2.2]Cryptand binds K+ as an octadentate N2O6 ligand. The resulting...
5 KB (304 words) - 14:12, 29 February 2024
use in the semiconductor industry is produced by the decomposition of diborane at high temperatures and then further purified by the zone melting or Czochralski...
127 KB (13,477 words) - 16:02, 23 September 2024
alloy (Na + Pb) Zinc amalgam (Zn(Hg)) (reagent for Clemmensen reduction) Diborane Sodium borohydride (Na BH4) Ferrous compounds that contain the Fe2+ ion...
15 KB (1,903 words) - 19:02, 8 July 2024
diboron tetrachloride 13701-67-2 B2F4 diboron tetrafluoride 13965-73-6 B2H6 diborane 19287-45-7 B2Mg magnesium diboride 12007-25-9 B2H2Se3 diboron triselenide...
183 KB (107 words) - 22:11, 6 September 2024