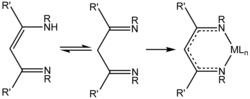
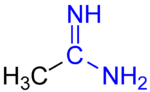
Diimines are organic compounds containing two imine (RCH=NR') groups. Common derivatives are 1,2-diimines and 1,3-diimines. These compounds are used as...
5 KB (529 words) - 04:10, 15 August 2024
Diimide, also called diazene or diimine, is a compound having the formula HN=NH. It exists as two geometric isomers, E (trans) and Z (cis). The term diazene...
8 KB (606 words) - 05:41, 29 August 2024
to a variety of bulky ligands. Condensation with glyoxal gives the 1,2-diimine ligands. An example is glyoxal-bis(mesitylimine), a yellow solid that is...
3 KB (231 words) - 15:22, 10 July 2024
discovered in the 1980-1990s. Nickel(II) and palladium(II) complexes of α-diimine ligands were known to efficiently catalyze polymerization of alkenes. They...
13 KB (1,433 words) - 21:25, 4 February 2024
some cases, HNacNacs also serve as charge-neutral 1,3-diimine ligands. NacNac ligands are diimine analogues of acetylacetonate ligands. An intermediate...
5 KB (437 words) - 21:49, 2 February 2024
molecules condense with the two carbonyl groups to give a diimine: In the second stage, this diimine condenses with the aldehyde: However, the actual reaction...
4 KB (357 words) - 15:50, 26 September 2024
a cyclic [3,3]-sigmatropic rearrangement occurs producing a diimine. The resulting diimine forms a cyclic aminoacetal (or aminal), which under acid catalysis...
9 KB (793 words) - 14:46, 26 September 2024
horseradish peroxidase. The resulting one-electron oxidation product is a diimine-diamine complex, which causes the solution to take on a blue colour, and...
6 KB (469 words) - 09:51, 24 January 2024
post-metallocene catalyst designs Catalyst supported by charge-neutral alpha-diimine ligands. Catalyst supported by highly electron-withdrawing substituted...
8 KB (958 words) - 21:54, 26 June 2022
the diamine condenses with 4-Trifluoromethylbenzaldehyde to give to the diimine. The salen ligands, some of which are used in catalysis, are derived from...
14 KB (1,116 words) - 18:18, 30 November 2024
The enol is about 1 kcal/mol more stable than the diketo form. Numerous diimine and dioxime ligands have been prepared from this diketone. It also condenses...
2 KB (145 words) - 22:10, 11 July 2024
1,1'-Diaminoferrocene has been incorporated into various diamide and diimine ligands, which form catalysts that exhibit redox switching. Fabbrizzi,...
2 KB (231 words) - 13:45, 13 August 2023
multiple names: authors list (link). Mashima, Kazushi (2020). "Redox-Active α-Diimine Complexes of Early Transition Metals: From Bonding to Catalysis". Bulletin...
7 KB (885 words) - 14:45, 24 November 2024
synthesized by condensation of 2,4,6-trimethylaniline and glyoxal to give the diimine. In the presence of acid, the resulting glyoxal-bis(mesitylimine) condenses...
5 KB (383 words) - 08:52, 2 November 2024
Quinone imine – where one O is replaced by N, illustrated by NAPQI Quinone diimine – where both O's are replaced by N's, illustrated by the antiseptic ambazone...
15 KB (1,742 words) - 15:28, 23 May 2024
are chemical compounds of the formula S(NR)2. Structurally, they are the diimine of sulfur dioxide. The parent member, S(NH)2, is of only theoretical interest...
4 KB (454 words) - 04:25, 14 November 2024
Paul J. (2018). "Synthesis and Electronic Structure Diversity of Pyridine(diimine)iron Tetrazene Complexes". Inorganic Chemistry. 57 (16): 9634–9643. doi:10...
3 KB (245 words) - 02:29, 17 December 2024
n-butyllithium is a Stork enamine / 1,2-addition cascade reaction: Iron[pyridine(diimine)] catalysts contain a redox active ligand in which the central iron atom...
12 KB (1,317 words) - 23:37, 6 December 2024
4-diaminobenzene derivative to the quinone state. 2) Reaction of this diimine with a coupler compound (more detail below). 3) Oxidation of the resulting...
37 KB (4,755 words) - 16:31, 1 November 2024
of monomer was added (5). α-diimine chelate initiators α-diimine chelate initiators are characterized by having a diimine chelating ancillary ligand structure...
37 KB (4,445 words) - 05:32, 3 June 2024
of the spectroscopy, photochemistry and electrochemistry of [M(CO)4(α-diimine)] complexes, M=Cr, Mo, W" Antonín Vlcek Coord. Chem. Rev. 230 (2002) 225-242...
4 KB (448 words) - 17:46, 21 September 2021
1,1'-Diaminoferrocene has been incorporated into various diamide and diimine ligands, which form catalysts that exhibit redox switching. Shafir, Alexandr;...
3 KB (238 words) - 23:52, 10 December 2024
aminoxyl radicals and was first thought to degrade only to the quinone diimine, but has since been understood to continue to oxidize to quinones, amongst...
16 KB (1,659 words) - 04:37, 7 October 2024
coordination chemistry. Oxidation of metal-phenylenediamine complexes affords the diimine derivatives. OPD condenses with salicylaldehyde to give chelating Schiff...
9 KB (528 words) - 22:10, 31 October 2024
Amidines are also prepared by the addition of organolithium reagents to diimines, followed by protonation or alkylation. Amidines are much more basic than...
8 KB (857 words) - 14:14, 24 December 2024
yellow solid that is soluble in organic solvents. It is classified as a diimine ligand. It is used in coordination chemistry and homogeneous catalysis...
3 KB (192 words) - 20:02, 19 August 2022
Diiminopyridines (DIP, also known a pyridine diimines, PDIs) are a class of diimine ligands. They featuring a pyridine nucleus with imine sidearms appended...
11 KB (1,250 words) - 10:03, 1 August 2024
subsequently undergoes elimination of p-toluenesulfinic acid and decomposes via a diimine intermediate 3 to the corresponding hydrocarbon. A slight variation of...
40 KB (4,211 words) - 08:09, 12 October 2024
Volumes, vol. 7, p. 41. Park, C. H.; Simmons, H. E. (1974). "Macrocyclic Diimines: 1,10-Diazacyclooctadecane". Organic Syntheses. 54: 88; Collected Volumes...
35 KB (3,057 words) - 14:36, 12 July 2024
sodium acetate solution produces primary sulfonamides in very good yields. Diimine can formed in situ from hydroxylamine-O-sulfonic acid respectively...
17 KB (1,606 words) - 08:45, 2 November 2024