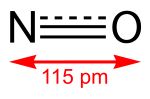
Lithium monoxide anion (LiO−) is a superbase existing in the gas phase. It was the strongest known base until 2008, when the isomeric diethynylbenzene...
3 KB (309 words) - 05:45, 10 December 2023
lithium carbonate: Li 2O + CO 2 → Li 2CO 3 The oxide reacts slowly with water, forming lithium hydroxide: Li 2O + H 2O → 2LiOH Lithium monoxide anion...
7 KB (480 words) - 18:59, 8 March 2024
Lithium hydroxide is an inorganic compound with the formula LiOH. It can exist as anhydrous or hydrated, and both forms are white hygroscopic solids....
14 KB (1,096 words) - 12:21, 25 August 2024
Methyl group (redirect from Methyl anion)
about 252.2 ± 3.3 kJ/mol. It is a powerful superbase; only the lithium monoxide anion (LiO−) and the diethynylbenzene dianions are known to be stronger...
12 KB (1,409 words) - 12:42, 4 October 2024
it appears that the carbonite ion reacts with excess carbon monoxide to form an anion with the ketene structure, O=C=C(−O−)2. Infrared spectroscopy...
5 KB (470 words) - 05:06, 25 September 2024
Para-diethynylbenzene dianion (C6H4(C2)2)2− (third strongest superbase) Lithium monoxide anion (LiO−) was considered the strongest superbase before diethynylbenzene...
26 KB (3,041 words) - 06:18, 8 August 2024
Diethynylbenzene dianion (category Anions)
function as superbases better than helonium does as a superacid. Lithium monoxide anion Poad, Berwyck L. J.; Reed, Nicholas D.; Hansen, Christopher S.;...
5 KB (486 words) - 15:04, 7 September 2024
Alkali metal (redirect from Lithium family)
boron–boron bonding in the lithium borides changes from following Wade's rules to forming Zintl anions like the rest of group 13. Lithium and sodium react with...
214 KB (23,508 words) - 15:30, 10 October 2024
Tetrakis(3,5-bis(trifluoromethyl)phenyl)borate (redirect from Kobayashi's anion)
Tetrakis[3,5-bis(trifluoromethyl)phenyl]borate is an anion with chemical formula [{3,5-(CF3)2C6H3}4B]−, which is commonly abbreviated as [BArF4]−, indicating...
11 KB (1,061 words) - 05:49, 25 June 2024
including silicon oxycarbide, silicon monoxide or silicon nitride. The first laboratory experiments with lithium-silicon materials took place in the early...
32 KB (3,566 words) - 05:23, 19 September 2024
Trisulfur (redirect from Trisulfur radical anion)
copper and gold in hydrothermal fluids. Lithium hexasulfide (which contains S−6, another polysulfide radical anion) with tetramethylenediamine solvation...
13 KB (1,331 words) - 21:09, 26 August 2024
introduced a new catalyst system. The catalytically active species is the anion cis-[Rh(CO)2I2]− (top of scheme). The first organometallic step is the oxidative...
5 KB (498 words) - 02:44, 15 July 2024
symmetric deltate anion, C3O2−3. The first deltate salts (of lithium and potassium) were described in 1976, also by Eggerding and West. Lithium deltate Li2C3O3...
5 KB (505 words) - 15:25, 22 November 2023
Nitric oxide (redirect from Nitrogen monoxide)
Nitric oxide (nitrogen oxide or nitrogen monoxide) is a colorless gas with the formula NO. It is one of the principal oxides of nitrogen. Nitric oxide...
31 KB (2,970 words) - 14:41, 2 September 2024
and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion. Carboxylic acids are commonly identified by their trivial names. They often...
26 KB (2,619 words) - 13:56, 13 October 2024
flame is dependent on the metal cation; the anion of the salt has very little direct influence. The anions however influence the flame temperature, both...
14 KB (573 words) - 19:54, 26 July 2024
(N≡N), the second strongest bond in any diatomic molecule after carbon monoxide (CO), dominates nitrogen chemistry. This causes difficulty for both organisms...
105 KB (12,228 words) - 16:47, 4 October 2024
Br(II) is known to occur in bromine monoxide radical; see Kinetics of the bromine monoxide radical + bromine monoxide radical reaction Colarusso, P.; Guo...
46 KB (8,729 words) - 23:27, 12 October 2024
oxides, such as dicarbon monoxide (C2O), oxalic anhydride (C2O4), and carbon trioxide (CO3). There are several oxocarbon anions, negative ions that consist...
13 KB (995 words) - 12:40, 1 July 2024
deprotonation at low temperature (−78 °C) with lithium diisopropylamide as the base to form a carbamoyl anion stable at these temperatures. In biochemistry...
18 KB (1,913 words) - 12:46, 27 August 2024
containing borate and oxalate anions. Where the oxalate group is bound to the borate via oxygen, a more condensed anion is formed that balances less cations...
11 KB (655 words) - 08:31, 15 June 2024
electropositive elements, such as lithium and sodium, the carbon ligand exhibits carbanionic character, but free carbon-based anions are extremely rare, an example...
31 KB (3,157 words) - 20:03, 24 September 2024
Copper(II) oxide (redirect from Copper monoxide)
Na2[Cu(OH)4] It can also be reduced to copper metal using hydrogen, carbon monoxide, and carbon: CuO + H2 → Cu + H2O CuO + CO → Cu + CO2 2 CuO + C → 2Cu +...
10 KB (829 words) - 15:20, 14 September 2024
Caesium monoxide or caesium oxide is a chemical compound with the chemical formula Cs2O. It is the simplest and most common oxide of the caesium. It forms...
5 KB (275 words) - 10:51, 14 September 2024
Oxocarbon (section Linear carbon monoxides)
carbon (≡C− ). The last two are found in carbon monoxide, −C≡O+. Negative oxygen occurs in most oxocarbon anions. One family of carbon oxides has the general...
34 KB (3,238 words) - 20:46, 20 June 2024
Li+ cations and Br− anions; thus, it is called lithium bromide. The compound BaO, which is composed of Ba2+ cations and O2− anions, is referred to as barium...
23 KB (2,775 words) - 07:16, 7 August 2024
streamlined the process using lithium chloride and potassium chloride with electrolysis to produce lithium and lithium hydroxide. During the later years...
50 KB (5,830 words) - 14:47, 4 October 2024
Sodium oxide (redirect from Sodium monoxide)
crystallise in the antifluorite structure. In this motif the positions of the anions and cations are reversed relative to their positions in CaF2, with sodium...
7 KB (542 words) - 05:02, 12 September 2024
Potassium oxide (redirect from Potassium monoxide)
crystallises in the antifluorite structure. In this motif the positions of the anions and cations are reversed relative to their positions in CaF2, with potassium...
7 KB (549 words) - 09:43, 22 June 2024
salt of this anion is one of the components of Liebig's so-called "potassium carbonyl", the product of the reaction of carbon monoxide with potassium...
8 KB (692 words) - 16:07, 13 July 2024