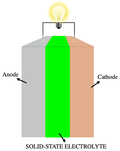
Lithium sulfide is the inorganic compound with the formula Li2S. It crystallizes in the antifluorite motif, described as the salt (Li+)2S2−. It forms...
5 KB (211 words) - 03:04, 15 July 2024
Harder, A.; Dauth, B. (1934). "Gitterstruktur der Oxyde, Sulfide, Selenide und Telluride des Lithiums, Natriums und Kaliums". Zeitschrift für Elektrochemie...
7 KB (480 words) - 18:59, 8 March 2024
Harder, A; Dauth, B. (1934). "Gitterstruktur der oxyde, sulfide, selenide und telluride des lithiums, natriums und kaliums". Z. Elektrochem. Angew. Phys....
13 KB (1,122 words) - 14:50, 14 August 2024
Solid-state battery (redirect from Solid-state lithium-ion battery)
electric vehicles. Solid-state batteries can use metallic lithium for the anode and oxides or sulfides for the cathode, increasing energy density. The solid...
84 KB (8,912 words) - 00:36, 3 October 2024
+ H2 though above 50 °C the product is lithium sulfide instead.: 9 LiH reacts with acetylene to form lithium carbide and hydrogen. With anhydrous organic...
19 KB (1,932 words) - 20:03, 23 July 2024
Anode-free battery (category Lithium-ion batteries)
reactions with flammable organic electrolytes. By contrast, lithium sulfide can reach 67% lithium. When fully lithiated, the cathode experiences negligible...
9 KB (1,061 words) - 15:03, 4 August 2024
(2013). "Recent development of sulfide solid electrolytes and interfacial modification for all-solid-state rechargeable lithium batteries". Journal of Asian...
207 KB (21,818 words) - 02:09, 10 October 2024
Potassium sulfide is an inorganic compound with the formula K2S. The colourless solid is rarely encountered, because it reacts readily with water, a reaction...
5 KB (297 words) - 00:27, 25 September 2023
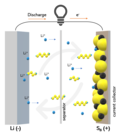
lithium–sulfur battery (Li–S battery) is a type of rechargeable battery. It is notable for its high specific energy. The low atomic weight of lithium...
59 KB (5,626 words) - 19:53, 17 September 2024
– FeLiO4P Lithium nitrate – LiNO3 Lithium sulfide – Li2S Lithium sulfite – Li2SO3 Lithium sulfate – Li2SO4 Lithium superoxide – LiO2 Lithium hexafluorophosphate...
119 KB (8,735 words) - 14:26, 16 September 2024
Rb_{2}S+H_{2}O} } Rubidium sulfide has a cubic crystal similar to lithium sulfide, sodium sulfide and potassium sulfide, known as the anti-fluorite structure...
4 KB (286 words) - 12:47, 25 January 2022
chloride with H2S, reducing chromium(III) sulfide with hydrogen, or by double replacement reaction of lithium sulfide with chromium(II) chloride. Cr + S ⟶...
5 KB (474 words) - 21:11, 16 January 2024
chloromethyl methyl sulfide: SO2Cl2 + (CH3)2S → SO2 + HCl + ClCH2SCH3 Like other methylthio compounds, DMS is deprotonated by butyl lithium: CH3CH2CH2CH2Li...
21 KB (2,081 words) - 16:41, 9 October 2024
pyridine, triethylamine, or 2,6-lutidine) in dichloromethane TMSCl and lithium sulfide (Li2S) in acetonitrile TMS groups are susceptible to cleavage upon...
9 KB (1,016 words) - 00:04, 19 July 2024
S2− → 1/x[SnS2− 3] x. Tin (IV) sulfide has various uses in electrochemistry. It can be used in anodes of lithium-ion batteries, where an intercalation...
8 KB (658 words) - 19:12, 12 September 2024
Lithium metal batteries are primary batteries that have metallic lithium as an anode. The name intentionally refers to the metal as to distinguish them...
51 KB (4,349 words) - 20:46, 30 September 2024
investigations claimed to prepare auric sulfide by the reaction of lithium tetrachloroaurate with hydrogen sulfide: 2 Li[AuCl4] + 3 H2S → Au2S3 + 2 LiCl...
4 KB (280 words) - 10:21, 30 December 2023
cesium sulfide emits rotten egg smelling hydrogen sulfide. Similar to sodium sulfide, anhydrous cesium sulfide can be produced by reacting cesium and sulfur...
4 KB (256 words) - 22:37, 12 April 2024
time at Stanford University, he used a layered titanium(IV) sulfide as cathode and lithium metal as anode. However, this setup proved impractical. Titanium...
54 KB (5,926 words) - 10:33, 7 October 2024
lithium carbonate 554–13–2 Li2MoO4 lithium molybdate 13568–40–6 Li2O lithium oxide 12057–24–8 Li2O2 lithium peroxide 12031–80–0 Li2S lithium sulfide 12136–58–2...
139 KB (120 words) - 17:07, 15 July 2024
Alkali metal (redirect from Lithium family)
The alkali metals consist of the chemical elements lithium (Li), sodium (Na), potassium (K), rubidium (Rb), caesium (Cs), and francium (Fr). Together...
214 KB (23,508 words) - 15:30, 10 October 2024
2012, CNMS produced a lithium-sulfide battery with a theoretical energy density three to five times greater than existing lithium ion batteries. ORNL provides...
55 KB (5,294 words) - 18:12, 18 August 2024
Pyrite (category Sulfide minerals)
as fool's gold, is an iron sulfide with the chemical formula FeS2 (iron (II) disulfide). Pyrite is the most abundant sulfide mineral. Pyrite's metallic...
43 KB (4,496 words) - 06:44, 8 October 2024
decomposition. In the carbon-free battery, the SEI stabilized quickly. Lithium phosphorus sulfide chloride forms less-reactive products. Li 6 PS 5 Cl ⟶ Li 2 S +...
8 KB (954 words) - 19:13, 5 February 2024
Lithium selenide is an inorganic compound that formed by selenium and lithium. It is a selenide with a chemical formula Li2Se. Lithium selenide has the...
7 KB (535 words) - 23:06, 11 May 2024
Organolithium reagent (redirect from Alkyl lithium reagent)
substrate that can be reduced with lithium metal to generate alkyllithium reagents is sulfides. Reduction of sulfides is useful in the formation of functionalized...
55 KB (5,971 words) - 20:35, 22 July 2024
Glass battery (redirect from Lithium glass battery)
electrolyte and lithium or sodium metal electrodes. The battery was invented by John B. Goodenough, inventor of the lithium cobalt oxide and lithium iron phosphate...
13 KB (1,447 words) - 03:41, 31 July 2024
Li2NbO3 lithium metaniobate 12031-63-9 Li2N2O2 lithium hyponitrite Li2O lithium oxide 12057-24-8 Li2O2 lithium peroxide 12031-80-0 Li2S lithium sulfide 12136-58-2...
183 KB (107 words) - 22:11, 6 September 2024
Sulfur (section Metal sulfides)
sometimes found in pure, native form, sulfur on Earth usually occurs as sulfide and sulfate minerals. Being abundant in native form, sulfur was known in...
99 KB (11,047 words) - 03:21, 28 September 2024
Dibenzothiophene (redirect from Diphenylene sulfide)
Dibenzothiophene (DBT, diphenylene sulfide) is the organosulfur compound consisting of two benzene rings fused to a central thiophene ring. It has the...
5 KB (237 words) - 00:38, 8 July 2024