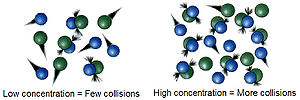
Molar concentration (also called molarity, amount concentration or substance concentration) is a measure of the concentration of a chemical species, in...
11 KB (1,388 words) - 01:48, 26 August 2024
be distinguished: mass concentration, molar concentration, number concentration, and volume concentration. The concentration can refer to any kind of...
9 KB (1,251 words) - 01:15, 30 May 2024
The molar conductivity of an electrolyte solution is defined as its conductivity divided by its molar concentration. Λ m = κ c , {\displaystyle \Lambda...
11 KB (1,376 words) - 17:32, 28 March 2024
Amount of substance (redirect from Molar quantity)
the molar concentration ( c {\displaystyle c} ) (also called amount of substance concentration, amount concentration, or substance concentration, especially...
29 KB (3,578 words) - 02:25, 24 August 2024
Molality (section Molar concentration (molarity))
mixed solvent. The term molality is formed in analogy to molarity which is the molar concentration of a solution. The earliest known use of the intensive...
21 KB (5,000 words) - 04:44, 22 July 2024
Mass fraction (chemistry) (section Molar concentration)
}}.} The relation to molar concentration is like that from above substituting the relation between mass and molar concentration: w i = ρ i ρ = c i M i...
5 KB (835 words) - 15:32, 30 May 2024
Mole fraction (redirect from Molar fraction)
In chemistry, the mole fraction or molar fraction, also called mole proportion or molar proportion, is a quantity defined as the ratio between the amount...
10 KB (1,839 words) - 00:48, 21 July 2024
conversion to molar concentration ci is given by: c i = ρ i M i {\displaystyle c_{i}={\frac {\rho _{i}}{M_{i}}}} where Mi is the molar mass of constituent...
8 KB (1,115 words) - 14:06, 31 May 2024
and the concentration of the species, according to the Beer–Lambert law A = ε c ℓ , {\displaystyle A=\varepsilon c\ell ,} where ε is the molar absorption...
6 KB (760 words) - 18:58, 26 June 2024
Reference ranges for blood tests (redirect from Serum concentration)
substance concentrations from the molar to the mass concentration scale above are made as follows: Numerically: molar concentration × molar mass = mass...
108 KB (6,105 words) - 10:44, 2 July 2024
magnitude of molar concentration. Source values are parenthesized where unit conversions were performed. M denotes the non-SI unit molar: 1 M = 1 mol/L...
13 KB (729 words) - 11:28, 8 July 2024
For example: glucose has n of 1, while NaCl has n of 2; C is the molar concentration of the solute; the index i represents the identity of a particular...
11 KB (1,360 words) - 19:20, 24 June 2024
to 360g/L), or between 4.81 and 5.58 mmol/L. It is thus a mass or molar concentration. Still, many instances measure MCHC in percentage (%), as if it were...
5 KB (596 words) - 16:00, 15 January 2024
In chemistry, the equivalent concentration or normality (N) of a solution is defined as the molar concentration ci divided by an equivalence factor or...
5 KB (595 words) - 16:43, 9 July 2024
Number density (redirect from Number concentration)
sometimes called concentration, although usually concentration is expressed as a number of moles per unit volume (and thus called molar concentration). For any...
7 KB (809 words) - 06:17, 22 April 2024
PH (redirect from Hydrogen-ion concentration)
-\log _{10}([{\ce {H+}}]/{\ce {M}})} where [H+] is the equilibrium molar concentration of H+ (M = mol/L) in the solution. At 25 °C (77°F), solutions with...
52 KB (6,462 words) - 06:34, 26 August 2024
IC50 (redirect from Half maximal inhibitory concentration)
cell receptor or microbe. IC50 values are typically expressed as molar concentration. IC50 is commonly used as a measure of antagonist drug potency in...
10 KB (1,084 words) - 20:25, 28 August 2024
In chemistry, the molar mass (M) (sometimes called molecular weight or formula weight, but see related quantities for usage) of a chemical compound is...
19 KB (2,842 words) - 14:49, 26 August 2024
Thermodynamic activity (redirect from Effective concentration)
the gas phase), molality bi, mass fraction wi, molar concentration (molarity) ci or mass concentration ρi: a i x = γ x , i x i , a i b = γ b , i b i...
21 KB (2,982 words) - 18:11, 21 April 2024
react, 2 mol of water (H2O) form. The concentration of a solution is commonly expressed by its molar concentration, defined as the amount of dissolved substance...
26 KB (3,298 words) - 15:24, 23 August 2024
colligative property, meaning that the osmotic pressure depends on the molar concentration of the solute but not on its identity. Osmosis is a vital process...
22 KB (2,757 words) - 14:22, 13 July 2024
Molar (unit), a unit of concentration equal to 1 mole per litre Molar quantities, such as molar mass, molar volume, etc. El Molar, Tarragona, a village...
882 bytes (163 words) - 13:42, 8 August 2024
per mass density Molar extinction coefficient, how strongly a substance absorbs light at a given wavelength, per molar concentration Optical extinction...
756 bytes (116 words) - 20:48, 5 March 2023
osmotic pressure, i is the dimensionless van 't Hoff index, c is the molar concentration of solute, R is the ideal gas constant, and T is the absolute temperature...
11 KB (1,559 words) - 01:06, 22 June 2024
kg). NA is the Avogadro constant. [A] is molar concentration of A in unit mol⋅L−1. [B] is molar concentration of B in unit mol⋅L−1. Z can be converted...
22 KB (3,224 words) - 17:23, 31 July 2024
depends solely on the ratio of the molar concentrations of the weak acid to the weak base. The higher the concentration of the weak acid in the solution...
21 KB (2,420 words) - 13:47, 7 August 2024
solvent, it is equal to the ratio of its molar concentration in the stationary phase to its molar concentration in the mobile phase, also approximating...
3 KB (362 words) - 01:12, 13 August 2023
In polymer chemistry, the molar mass distribution (or molecular weight distribution) describes the relationship between the number of moles of each polymer...
10 KB (1,391 words) - 17:17, 4 February 2024
titrations. The concentrations of standard solutions are normally expressed in units of moles per litre (mol/L, often abbreviated to M for molarity), moles per...
6 KB (688 words) - 18:52, 16 April 2024
that the product of molar concentration of calcium ions (moles of dissolved Ca2+ per liter of solution) with the molar concentration of dissolved CO2−3...
79 KB (7,564 words) - 18:12, 8 August 2024