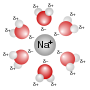
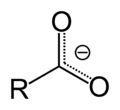
A polar aprotic solvent is a solvent that lacks an acidic proton and is polar. Such solvents lack hydroxyl and amine groups. In contrast to protic solvents...
3 KB (140 words) - 17:22, 13 October 2023
use of polar protic solvents favors the SN1 reaction mechanism, while polar aprotic solvents favor the SN2 reaction mechanism. These polar solvents are capable...
45 KB (3,723 words) - 01:17, 15 November 2024
Dimethyl sulfoxide (category Solvents)
an important polar aprotic solvent that dissolves both polar and nonpolar compounds and is miscible in a wide range of organic solvents as well as water...
38 KB (3,969 words) - 18:58, 5 November 2024
via hydrogen bonding. Water is the most common protic solvent. Conversely, polar aprotic solvents cannot donate protons but still have the ability to dissolve...
3 KB (165 words) - 23:54, 21 December 2023
Propylene carbonate (category Ester solvents)
propylene glycol. This colorless and odorless liquid is useful as a polar, aprotic solvent. Propylene carbonate is chiral, but is used as the racemic mixture...
8 KB (733 words) - 10:23, 17 October 2024
SN2 reaction (section Solvent)
water, and I− is a better nucleophile than Br− (in polar protic solvents). In a polar aprotic solvent, nucleophilicity increases up a column of the periodic...
21 KB (2,555 words) - 19:33, 4 November 2024
Sulfolane (category Solvents)
Company in the 1960s as a solvent to purify butadiene. Sulfolane is a polar aprotic solvent, and it is miscible with water. Sulfolane is classified as a sulfone...
11 KB (1,053 words) - 22:17, 17 April 2024
1,3-Dimethyl-2-imidazolidinone (category Solvents)
high-boiling polar aprotic solvent. It is colourless, highly polar solvent has high thermal and chemical stability. It is a homolog of the related solvent DMPU...
3 KB (174 words) - 09:00, 2 September 2024
Acetonitrile (category Solvents)
mainly as a byproduct of acrylonitrile manufacture. It is used as a polar aprotic solvent in organic synthesis and in the purification of butadiene. The N≡C−C...
21 KB (1,789 words) - 03:04, 2 November 2024
from a protic solvent to an aprotic solvent. This difference arises from acid/base reactions between protic solvents (not aprotic solvents) and strong nucleophiles...
17 KB (1,904 words) - 19:14, 18 October 2024
Acetone (category Ketone solvents)
[needs update] A variety of organic reactions employ acetone as a polar, aprotic solvent, e.g. the Jones oxidation. Because acetone is cheap, volatile, and...
47 KB (4,849 words) - 16:17, 7 November 2024
Tetraethylene glycol dimethyl ether (TEGDME or tetraglyme) is a polar aprotic solvent with excellent chemical and thermal stability. Its high boiling...
5 KB (286 words) - 04:15, 21 October 2022
solvation sheath is the solvent interface of any chemical compound or biomolecule that constitutes the solute in a solution. When the solvent is water it is called...
5 KB (642 words) - 09:58, 7 January 2024
2'-dipyridyldisulfide and triphenylphosphine as reagents and runs in polar aprotic solvent under mild conditions. The hydroxy acid first reacts with the 2...
4 KB (408 words) - 16:13, 26 September 2024
hydrocarbons produced in steam cracking by extractive distillation using a polar aprotic solvent such as acetonitrile, N-methyl-2-pyrrolidone, furfural, or dimethylformamide...
31 KB (3,158 words) - 22:17, 11 November 2024
and alkoxide ions, but stronger than that of halide anions (in a polar aprotic solvent, though there are other effects such as solubility of the ion)....
5 KB (604 words) - 21:59, 18 August 2024
Dihydrolevoglucosenone (category Solvents)
cycloalkanone which is a waste derived and fully biodegradable aprotic dipolar solvent. It is an environmentally friendly alternative to dimethylformamide...
15 KB (1,407 words) - 13:03, 30 October 2024
triethylamine to form amide bonds. Typically DMF is used as solvent, although other polar aprotic solvents can also be used. HATU was first reported by Louis A...
10 KB (895 words) - 14:49, 28 October 2024
piperidine. It can be used as a polar aprotic solvent, with better hydrocarbon solubility than other amide solvents such as dimethylformamide (DMF)....
3 KB (157 words) - 20:03, 27 December 2022
Piperidine (category Amine solvents)
Piperidine is used as a solvent and as a base. The same is true for certain derivatives: N-formylpiperidine is a polar aprotic solvent with better hydrocarbon...
17 KB (1,471 words) - 09:57, 23 October 2024
1,2-Butylene carbonate (category Solvents)
specifically 4-ethyl-1,3-dioxolan-2-one. 1,2-Butylene carbonate is a polar aprotic solvent, which has been considered for electric battery applications (as...
2 KB (133 words) - 14:07, 17 June 2020
development of bio-based solvents: a case study on methyl(2,2-dimethyl-1,3-dioxolan-4-yl)methyl carbonate as an alternative aprotic solvent" (PDF). Faraday Discussions...
48 KB (5,170 words) - 05:01, 11 November 2024
reaction generally takes place at room temperature. A variety of polar aprotic solvents can be used. Because the reaction is mild, esters can be obtained...
4 KB (361 words) - 18:33, 26 September 2024
DMPU (category Solvents)
N′-Dimethylpropyleneurea (DMPU) is a cyclic urea sometimes used as a polar, aprotic organic solvent. In 1985, Dieter Seebach showed that it is possible to replace...
4 KB (172 words) - 07:31, 20 November 2021
Dimethyl sulfoxide an organosulfur compound; an important polar aprotic solvent that dissolves both polar and nonpolar compounds Dioxane a heterocyclic organic...
15 KB (264 words) - 18:51, 14 August 2022
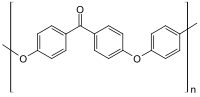
with sodium carbonate. The reaction is conducted around 300 °C in polar aprotic solvents - such as diphenyl sulfone. PEEK is a semicrystalline thermoplastic...
10 KB (1,007 words) - 16:32, 7 November 2024
soluble and fusible high-molecular-weight poly(amic acid) in a polar aprotic solvent such as NMP or N,N-dimethylacetamide. The poly(amic aicd) can then...
28 KB (3,709 words) - 07:02, 8 September 2024
generally not. As such it is commercially available as a solution in polar aprotic solvents such as THF and ether; however, for small scale use (less than 50 mmol)...
8 KB (776 words) - 23:57, 16 May 2024
nucleophiles. In polar, protic solvents, F− is the weakest nucleophile, and I− the strongest; this order is reversed in polar, aprotic solvents. Carbon nucleophiles...
17 KB (2,157 words) - 01:05, 29 October 2024
(protic and aprotic solvents). Water, the most commonly used solvent, is both polar and sustains hydrogen bonds. Salts dissolve in polar solvents, forming...
10 KB (1,290 words) - 16:31, 7 October 2024