chemistry, a reaction step of a chemical reaction is defined as: "An elementary reaction, constituting one of the stages of a stepwise reaction in which a...
810 bytes (91 words) - 19:09, 13 October 2024
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs. A chemical mechanism...
13 KB (1,686 words) - 06:29, 9 August 2024
solvent) a third step is required to complete the reaction. When the solvent is water, the intermediate is an oxonium ion. This reaction step is fast. Deprotonation:...
15 KB (1,970 words) - 03:54, 24 October 2024
Molecularity (redirect from Trimolecular reaction)
elementary (single-step) reaction and is equal to the sum of stoichiometric coefficients of reactants in the elementary reaction with effective collision...
8 KB (1,137 words) - 21:06, 1 October 2023
elimination reaction is a type of organic reaction in which two substituents are removed from a molecule in either a one- or two-step mechanism. The one-step mechanism...
14 KB (1,844 words) - 22:24, 27 May 2024
following slow reaction between the iodate and bisulfite: IO−3 + 3 HSO−3 → I− + 3 HSO−4 This first step is the rate determining step. Next, the iodate...
9 KB (991 words) - 14:03, 26 June 2024
chain reaction is a sequence of reactions where a reactive product or by-product causes additional reactions to take place. In a chain reaction, positive...
18 KB (2,580 words) - 11:04, 9 August 2024
elementary reaction is a chemical reaction in which one or more chemical species react directly to form products in a single reaction step and with a...
4 KB (479 words) - 09:23, 4 April 2024
Knoevenagel condensation (redirect from Knaevenagel reaction)
and the Feist–Benary furan synthesis all contain a Knoevenagel reaction step. The reaction also led to the discovery of CS gas. The Doebner modification...
7 KB (729 words) - 15:22, 28 August 2024
Polymerization (redirect from Polymerization reaction)
slowly. Long chains form only late in the reaction. Step-growth polymers are formed by independent reaction steps between functional groups of monomer...
14 KB (1,444 words) - 18:51, 4 August 2024
In biochemistry, a rate-limiting step is a reaction step that controls the rate of a series of biochemical reactions. The statement is, however, a misunderstanding...
11 KB (1,402 words) - 17:28, 13 October 2024
elimination step by reaction of the alkoxide with the palladium complex. In most cases the oxidative addition is the rate determining step of the catalytic...
34 KB (3,845 words) - 17:47, 26 September 2024
surface) associated with the reaction. In the formalism of transition-state theory the reaction coordinate for each reaction step is one of a set of curvilinear...
5 KB (579 words) - 12:16, 26 September 2024
In chemistry, a concerted reaction is a chemical reaction in which all bond breaking and bond making occurs in a single step. Reactive intermediates or...
2 KB (211 words) - 13:52, 19 October 2023
formed as the reaction product of an elementary step, from the reactants and/or preceding intermediates, but is consumed in a later step. It does not appear...
14 KB (1,828 words) - 17:06, 8 August 2024
reaction is often approximately determined by the slowest step, known as the rate-determining step (RDS or RD-step or r/d step) or rate-limiting step...
12 KB (1,736 words) - 20:02, 2 September 2024
in the rate-determining step. What distinguishes SN2 from the other major type of nucleophilic substitution, the SN1 reaction, is that the displacement...
21 KB (2,555 words) - 19:33, 4 November 2024
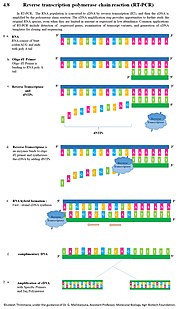
one-step or a two-step reaction. The difference between the two approaches lies in the number of tubes used when performing the procedure. The two-step reaction...
44 KB (5,656 words) - 23:37, 29 October 2024
Biosynthesis (category Biochemical reactions)
other compounds, usually as part of a multiple step reaction pathway. Two examples of this type of reaction occur during the formation of nucleic acids and...
61 KB (6,770 words) - 15:11, 20 August 2024
Claus process (redirect from Claus reaction)
converted to SO2. This ensures a stoichiometric reaction for the Claus reaction in the second catalytic step (see next section below). The separation of the...
16 KB (2,060 words) - 15:16, 18 August 2024
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration...
26 KB (3,716 words) - 19:46, 19 September 2024
biochemistry, the committed step (also known as the first committed step) is an effectively irreversible, enzyme-catalyzed reaction that occurs at a branch...
7 KB (673 words) - 18:57, 13 October 2024
The Heck reaction (also called the Mizoroki–Heck reaction) is the chemical reaction of an unsaturated halide (or triflate) with an alkene in the presence...
13 KB (1,355 words) - 19:29, 15 December 2023
Lindemann mechanism (category Reaction mechanisms)
some reaction steps. It breaks down an apparently unimolecular reaction into two elementary steps, with a rate constant for each elementary step. The...
11 KB (1,670 words) - 12:06, 27 June 2024
Hantzsch pyridine synthesis (category Ring forming reactions)
initial reaction product is a dihydropyridine which can be oxidized in a subsequent step to a pyridine. The driving force for this second reaction step is...
12 KB (1,230 words) - 14:25, 26 September 2024
Citrate–malate shuttle (category Biochemical reactions)
the two intermediates – short-lived chemicals that are generated in a reaction step and consumed entirely in the next – citrate and malate that carry the...
26 KB (2,998 words) - 13:33, 22 July 2024
well-established example is the termolecular step 2 I + H 2 → 2 HI in the hydrogen-iodine reaction. In cases where a termolecular step might plausibly be proposed, one...
17 KB (2,387 words) - 00:47, 18 October 2024
double bond, and a proton (H+) adds to the other. The reaction is highly exothermic. In the first step, the alkene acts as a nucleophile and attacks the proton...
6 KB (681 words) - 10:12, 7 July 2024
In electrochemistry, an electrochemical reaction mechanism is the step-by-step sequence of elementary steps, involving at least one outer-sphere electron...
9 KB (1,244 words) - 17:31, 1 May 2023
mechanism, a high extent of reaction is required to achieve high molecular weight. The easiest way to visualize the mechanism of a step-growth polymerization...
28 KB (3,709 words) - 07:02, 8 September 2024