conjugate bases of phosphorous acid (H3PO3). The corresponding salts, e.g. sodium phosphite (Na2HPO3) are reducing in character. The IUPAC recommended name for...
10 KB (832 words) - 18:34, 13 August 2024
Phasenbeziehungen und kristallographische Untersuchungen (Hydrates of sodium phosphite, phase relations and crystallographic studies)". Zeitschrift für Anorganische...
3 KB (133 words) - 09:02, 31 May 2023
Disodium phosphate (redirect from Sodium hydrogen phosphate)
hydrogen phosphate, or sodium phosphate dibasic, is an inorganic compound with the chemical formula Na2HPO4. It is one of several sodium phosphates. The salt...
7 KB (367 words) - 00:52, 15 May 2024
Sodium hypophosphite (NaPO2H2, also known as sodium phosphinate) is the sodium salt of hypophosphorous acid and is often encountered as the monohydrate...
5 KB (303 words) - 19:40, 26 August 2023
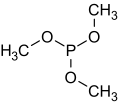
side reactions however. The use of sodium methoxide is superior: PCl3 + 3 NaOCH3 → P(OCH3)3 + 3 NaCl Trimethyl phosphite is susceptible to oxidation to trimethyl...
5 KB (434 words) - 00:50, 30 June 2024
The oxalate phosphites are chemical compounds containing oxalate and phosphite anions. They are also called oxalatophosphites or phosphite oxalates. Oxalate...
30 KB (1,506 words) - 16:01, 9 September 2023
Sodium tetraphenylborate is the organic compound with the formula NaB(C6H5)4. It is a salt, wherein the anion consists of four phenyl rings bonded to boron...
6 KB (631 words) - 00:31, 19 March 2024
Trimethylolpropane phosphite, C2H5C(CH2O)3P, is a phosphite ester used as a ligand in organometallic chemistry. Trimethylolpropane phosphite is sometimes abbreviated...
5 KB (364 words) - 14:51, 30 April 2023
Ceftolozane/tazobactam (redirect from Ceftolozane Sulfate and Tazobactam Sodium)
the coupling reaction were the use of a designed, electron-deficient phosphite ligand in tandem with the addition of an exogenous chloride scavenging...
23 KB (2,189 words) - 02:15, 16 July 2024
low-temperature argon matrix. Phosphite ozonides, (RO)3PO3, are used in the production of singlet oxygen. They are made by ozonizing a phosphite ester in dichloromethane...
7 KB (642 words) - 23:25, 28 February 2023
is an important industrial chemical, being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with...
18 KB (1,731 words) - 16:03, 2 September 2023
HPO 42− Organophosphinic acid Phosphine - PR3 Phosphine oxide - OPR3 Phosphite - P(OR)3 Phosphonate - OP(OR)2R Phosphate - OP(OR)3 Greenwood, Norman...
2 KB (183 words) - 10:15, 30 June 2024
The Perkow reaction is an organic reaction in which a trialkyl phosphite ester reacts with a haloketone to form a dialkyl vinyl phosphate and an alkyl...
4 KB (437 words) - 23:45, 5 April 2023
Trimethylolpropane phosphite Trimethyltin chloride Triphenyltin chloride Tris(2-chloroethyl)amine Valinomycin Vinyl acetate monomer Warfarin Warfarin sodium Xylylene...
10 KB (825 words) - 19:18, 7 August 2024
Abramov (1904–1968) in 1957. Electron-rich sources of phosphorus such as phosphites, phosphonites, and phosphinites may undergo nucleophilic addition to carbon...
10 KB (1,098 words) - 04:28, 23 June 2022
3'-Dimethylbenzidine (o-Tolidine) 1,1-Dimethylhydrazine Dimethyl hydrogen phosphite Dimethyl-p-toluidine 3,7-Dinitrofluoranthene 3,9-Dinitrofluoranthene 1...
15 KB (1,444 words) - 16:05, 28 August 2024
manufactures phosphorus oxychloride, phosphorus trichloride, and dimethyl phosphite, and phosphorus-based insecticides, herbicides and dyestuffs. As of June...
6 KB (475 words) - 03:56, 24 August 2024
written as O2S(OH)2; this is the molecule observed in the gas phase. The phosphite ion, PO3−3, is a strong base, and so always carries at least one proton...
18 KB (2,163 words) - 14:15, 13 August 2024
the IUPAC Phosphorous acid forms two types of esters: phosphite esters, e.g. triethyl phosphite (P(−O−CH2CH3)3), and phosphonate esters, e.g. diethyl...
45 KB (4,778 words) - 01:40, 26 August 2024
phosphonium salts, but by deprotonation not alkylation. Phosphites, sometimes called phosphite esters, have the general structure P(OR)3 with oxidation...
16 KB (1,730 words) - 17:17, 3 June 2024
OP(OR)R2 Phosphine – PR3 Phosphine oxide – OPR3 Phosphinite – P(OR)R2 Phosphite – P(OR)3 Phosphogypsum Phosphonate – OP(OR)2R Phosphonite – P(OR)2R Phosphorylation...
30 KB (2,574 words) - 15:28, 24 August 2024
the alcohol with sodium hydroxide or potassium hydroxide: ROH + CS2 + KOH → ROCS2K + H2O For example, sodium ethoxide gives sodium ethyl xanthate. Many...
10 KB (1,072 words) - 18:24, 2 June 2024
ethers and alcohols. Aqueous sodium hydroxide and carboxylic acids, even hydrophobic ones, react to yield water-soluble sodium salts. For example, enanthic...
26 KB (2,619 words) - 01:49, 26 August 2024
Silver Candid Indian Sons Sobbing Tears In Xcess Cease Bawling. Lithium, Sodium, Potassium, Rubidium, Caesium, Francium Little Nasty Kids Rub Cats Fur Little...
38 KB (2,685 words) - 22:28, 1 September 2024
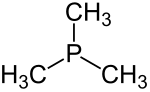
for phosphine, PH3. PMe3 can be prepared by the treatment of triphenyl phosphite with methylmagnesium chloride: 3 CH3MgCl + P(OC6H5)3 → P(CH3)3 + 3 C6H5OMgCl...
7 KB (548 words) - 09:15, 2 September 2024
contrasts to the corresponding anions of the lighter members of group 15, phosphite which has the structure HPO2−3 and nitrite, NO−2 which is bent. A number...
9 KB (992 words) - 20:54, 25 June 2024
represent recreational drugs. Organic nitrites are prepared from alcohols and sodium nitrite in sulfuric acid solution. They decompose slowly on standing, the...
5 KB (554 words) - 15:31, 1 September 2024
Ascorbic acid (C6H8O6) Reducing sugars, such as erythrose, see Aldose Phosphites, hypophosphites, and phosphorous acid Dithiothreitol (DTT) – used in biochemistry...
15 KB (1,903 words) - 19:02, 8 July 2024
faster than 1-chloropropane by a factor of 36,000. Halo ketones react with phosphites in the Perkow reaction. The halo group can be removed in reductive dehalogenation...
6 KB (670 words) - 13:34, 30 October 2023
environments as large deposits, particularly of nitratine, a major source of sodium nitrate. Nitrates are produced by a number of species of nitrifying bacteria...
32 KB (3,503 words) - 05:17, 28 August 2024