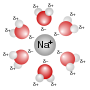
Solution (chemistry) (redirect from Solute)
mixture, a solute is a substance dissolved in another substance, known as a solvent. If the attractive forces between the solvent and solute particles...
14 KB (1,883 words) - 11:40, 17 June 2024
Solute pumping is a form of active transport of a solute through a cell membrane. Solute pumping allows a molecule that cannot regularly cross the lipid...
967 bytes (90 words) - 20:39, 3 July 2021
Solubility (redirect from Chemical solute)
substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such...
50 KB (6,544 words) - 02:47, 3 July 2024
solute concentration) to a region of low water potential (region of higher solute concentration), in the direction that tends to equalize the solute concentrations...
22 KB (2,757 words) - 14:22, 13 July 2024
The solute carrier (SLC) group of membrane transport proteins include over 400 members organized into 66 families. Most members of the SLC group are located...
35 KB (3,408 words) - 02:42, 30 November 2023
Osmotic pressure (redirect from Solute potential)
concentration of solute, R is the ideal gas constant, and T is the absolute temperature (usually in kelvins). This formula applies when the solute concentration...
11 KB (1,559 words) - 01:06, 22 June 2024
those properties of solutions that depend on the ratio of the number of solute particles to the number of solvent particles in a solution, and not on the...
13 KB (2,107 words) - 18:02, 9 July 2024
Solid solution strengthening (redirect from Solute strengthening)
Pauling's rules. Substitutional solid solution strengthening occurs when the solute atom is large enough that it can replace solvent atoms in their lattice...
19 KB (2,487 words) - 10:10, 19 May 2024
Chaotropic agent (redirect from Chaotropic solute)
macromolecules such as proteins and nucleic acids (e.g. DNA and RNA). Chaotropic solutes increase the entropy of the system by interfering with intermolecular interactions...
5 KB (604 words) - 19:13, 3 April 2024
solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles...
18 KB (2,345 words) - 15:28, 12 June 2024
Plasma osmolality (redirect from Blood solute)
osmoles (Osm) of solute per kilogram of solvent (osmol/kg or Osm/kg), osmolarity (with an "r") is defined as the number of osmoles of solute per liter (L)...
11 KB (1,269 words) - 12:26, 18 January 2024
Van 't Hoff factor (section Dissociated solutes)
Dutch chemist Jacobus Henricus van 't Hoff) is a measure of the effect of a solute on colligative properties such as osmotic pressure, relative lowering in...
4 KB (616 words) - 23:17, 7 May 2024
Osmotic concentration (section Types of solutes)
known as osmolarity, is the measure of solute concentration, defined as the number of osmoles (Osm) of solute per litre (L) of solution (osmol/L or Osm/L)...
11 KB (1,360 words) - 19:20, 24 June 2024
of the Solute:Sodium Symporter (SSS) Family (TC# 2.A.21) catalyze solute:Na+ symport. The SSS family is within the APC Superfamily. The solutes transported...
12 KB (1,408 words) - 04:23, 29 November 2023
Osmoprotectant (redirect from Compatible solutes)
Osmoprotectants or compatible solutes are small organic molecules with neutral charge and low toxicity at high concentrations that act as osmolytes and...
6 KB (710 words) - 22:12, 17 December 2023
Leaching is the process of a solute becoming detached or extracted from its carrier substance by way of a solvent. Leaching is a naturally occurring process...
17 KB (1,892 words) - 15:48, 9 May 2024
distribution of a solute between two immiscible solvents. This law was first given by Nernst who studied the distribution of several solutes between different...
2 KB (271 words) - 04:01, 25 April 2023
gradient (spatial derivative), or in simplistic terms the concept that a solute will move from a region of high concentration to a region of low concentration...
56 KB (7,886 words) - 14:28, 17 July 2024
molecules or solutes on either side, as well as the permeability of the membrane to each solute. Depending on the membrane and the solute, permeability...
17 KB (1,819 words) - 19:28, 3 July 2024
the Latin solvō, "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be...
44 KB (3,609 words) - 23:47, 14 June 2024
depends on the relative concentration of selective membrane-impermeable solutes across a cell membrane which determine the direction and extent of osmotic...
9 KB (979 words) - 21:26, 6 January 2024
can refer to any kind of chemical mixture, but most frequently refers to solutes and solvents in solutions. The molar (amount) concentration has variants...
9 KB (1,251 words) - 01:15, 30 May 2024
solvent. If w represents the mass fraction of the solute in solution, and assuming no dissociation of the solute, the molar mass is given by M = w K f Δ T ....
19 KB (2,813 words) - 09:38, 21 June 2024
electric potential. Kidney dialysis is the process of removing water, solutes and toxins from the blood of individuals with compromised kidney function...
681 bytes (115 words) - 15:20, 27 December 2023
boiling point than a pure solvent. This happens whenever a non-volatile solute, such as a salt, is added to a pure solvent, such as water. The boiling...
8 KB (1,054 words) - 00:03, 24 December 2023
cases, the substance added/present in smaller amounts is considered the solute, while the original substance present in larger quantity is thought of as...
19 KB (2,360 words) - 10:01, 20 June 2024
lysis, 'loosening or splitting') is the process of removing excess water, solutes, and toxins from the blood in people whose kidneys can no longer perform...
47 KB (5,495 words) - 22:59, 15 July 2024
chemistry, supersaturation occurs with a solution when the concentration of a solute exceeds the concentration specified by the value of solubility at equilibrium...
18 KB (2,164 words) - 00:01, 8 November 2023
measure of the concentration of a chemical species, in particular, of a solute in a solution, in terms of amount of substance per unit volume of solution...
11 KB (1,391 words) - 01:08, 16 April 2024
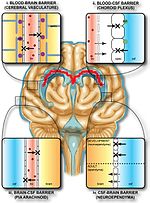
semipermeable border of endothelial cells that regulates the transfer of solutes and chemicals between the circulatory system and the central nervous system...
33 KB (3,721 words) - 15:09, 4 July 2024