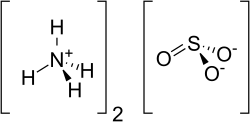
Sulfites or sulphites are compounds that contain the sulfite ion (systematic name: sulfate(IV) ion), SO2− 3. The sulfite ion is the conjugate base of bisulfite...
20 KB (1,829 words) - 04:22, 17 March 2025
Sodium sulfite (sodium sulphite) is the inorganic compound with the chemical formula Na2SO3. A white, water-soluble solid, it is used commercially as...
7 KB (594 words) - 16:03, 24 January 2025
Sulfite oxidase (EC 1.8.3.1) is an enzyme in the mitochondria of all eukaryotes, with exception of the yeasts.[citation needed] It oxidizes sulfite to...
13 KB (1,683 words) - 17:02, 1 March 2024
Sodium bisulfite (redirect from Sodium hydrogen sulfite)
Sodium bisulfite (or sodium bisulphite, sodium hydrogen sulfite) is a chemical mixture with the approximate chemical formula NaHSO3. Sodium bisulfite...
12 KB (1,032 words) - 15:28, 10 April 2025
Calcium sulfite, or calcium sulphite, is a chemical compound, the calcium salt of sulfite with the formula CaSO3·x(H2O). Two crystalline forms are known...
8 KB (744 words) - 00:06, 23 November 2024
Potassium sulfite is the inorganic compound with the formula K2SO3. It is the salt of potassium cation and sulfite anion. It is a white solid that is highly...
4 KB (303 words) - 16:17, 5 February 2025
The sulfite process produces wood pulp that is almost pure cellulose fibers by treating wood chips with solutions of sulfite and bisulfite ions. These...
13 KB (1,535 words) - 00:20, 25 January 2023
Bismuth sulfite agar is a type of agar media used to isolate Salmonella species. It uses glucose as a primary source of carbon. Bismuth and brilliant...
2 KB (131 words) - 03:58, 23 December 2023
Ammonium sulfite is the ammonium salt of sulfurous acid with the chemical formula (NH4)2SO3. Ammonium sulfite can be prepared by the reaction of ammonia...
6 KB (413 words) - 14:59, 12 August 2024
Dimethyl sulfite is a sulfite ester with the chemical formula (CH3O)2SO. Dimethyl sulfite is used as an additive in some polymers to prevent oxidation...
4 KB (323 words) - 00:49, 21 November 2024
Sulfur trioxide (redirect from Sulfite radical)
Sulfites CdSO3 K2SO3 Sulfates Ag2SO4 CaSO4 CuSO4 Cs2SO4 Er2(SO4)3 Eu2(SO4)3 HgSO4 K2SO4 KAl(SO4)2 NaAl(SO4)2 RaSO4 SnSO4 SrSO4 Ti(SO4)2 Tm2(SO4)3 Yb2(SO4)3...
18 KB (1,668 words) - 14:46, 4 March 2025
Potassium bisulfite (redirect from Potassium hydrogen sulfite)
Potassium bisulfite (or potassium hydrogen sulfite) is a chemical mixture with the approximately correctly mentioned formula chemical formula KHSO3. Potassium...
4 KB (242 words) - 16:17, 5 February 2025
Lithium sulfite, or lithium sulphite, is an ionic compound with the formula Li2SO3. Fujita, Yushi; Motohashi, Kota; Ding, Jiong; Tsukasaki, Hirofumi;...
2 KB (67 words) - 17:31, 31 October 2024
The topic of sulfite food and beverage additives covers the application of sulfites in food chemistry. "Sulfite" is jargon that encompasses a variety of...
19 KB (1,862 words) - 18:13, 3 March 2025
Sulfite reductases (EC 1.8.99.1) are enzymes that participate in sulfur metabolism. They catalyze the reduction of sulfite to hydrogen sulfide and water...
6 KB (603 words) - 13:01, 22 April 2024
Silver sulfite is the chemical compound with the formula Ag2SO3. This unstable silver compound when heated and/or in light it decomposes to silver dithionate...
4 KB (184 words) - 14:58, 12 August 2024
In enzymology, a sulfite dehydrogenase (EC 1.8.2.1) is an enzyme that catalyzes the chemical reaction sulfite + 2 ferricytochrome c + H2O ⇌ {\displaystyle...
2 KB (176 words) - 15:56, 26 August 2023
Caramel color (redirect from Sulfite ammonia caramel)
growth unless in a dilute solution. When reacted with sulfites, caramel color may retain traces of sulfite after processing. However, in finished food products...
19 KB (1,791 words) - 15:48, 14 April 2025
Calcium bisulfite (redirect from Calcium hydrogen sulfite)
Calcium bisulfite (calcium bisulphite or calcium hydrogen sulfite) is an inorganic compound which is the salt of a calcium cation and a bisulfite anion...
8 KB (824 words) - 11:32, 26 December 2024
A sulfite ester (also known as an organosulfite) is a functional group with the structure (RO)(R'O)SO. They are in principle the esters of sulfurous acid...
2 KB (238 words) - 09:51, 17 April 2023
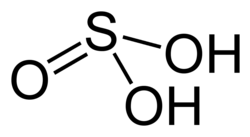
Sulfurous acid (category Sulfites)
ionization of diethyl sulfite. The conjugate bases of this elusive acid are, however, common anions, bisulfite (or hydrogen sulfite) and sulfite. Sulfurous acid...
6 KB (449 words) - 08:29, 20 March 2025
Magnesium sulfite is the magnesium salt of sulfurous acid with the formula MgSO 3. Its most common hydrated form has 6 water molecules making it a hexahydrate...
2 KB (104 words) - 03:27, 1 March 2025
Diethyl sulfite (C4H10O3S) is an ester of sulfurous acid. Among other properties, diethyl sulfite inhibits the growth of mold spores during grain storage...
2 KB (118 words) - 15:36, 19 August 2022
Bisulfite (redirect from Hydrogen sulfite)
is the ion HSO− 3. Salts containing the HSO− 3 ion are also known as "sulfite lyes". Sodium bisulfite is used interchangeably with sodium metabisulfite...
10 KB (1,061 words) - 17:10, 19 December 2024
Barium sulfite is the inorganic compound with the chemical formula BaSO3. It is a white powder that finds few applications. It is an intermediate in the...
3 KB (104 words) - 14:58, 12 August 2024
using mechanical, semi-chemical, or fully chemical methods (kraft and sulfite processes). The finished product may be either bleached or non-bleached...
15 KB (1,936 words) - 15:31, 24 March 2025
Isolated sulfite oxidase deficiency is a rare, fatal genetic disease caused by mutations to sulfite oxidase, which is needed to metabolize sulfites to sulfates...
97 KB (10,844 words) - 15:58, 6 April 2025
A sulfite sulfate is a chemical compound that contains both sulfite and sulfate anions [SO3]2− [SO4]2−. These compounds were discovered in the 1980s as...
17 KB (1,520 words) - 14:59, 12 August 2024
of lime results in a slurry of calcium sulfite (CaSO3) that must be disposed of. Fortunately, calcium sulfite can be oxidized to produce by-product gypsum...
26 KB (3,415 words) - 19:05, 5 March 2025
photographers add a pinch of sodium sulfite before dissolving the metol to prevent oxidation, but large amounts of sulfite in solution will make the metol...
18 KB (2,448 words) - 18:19, 4 April 2025