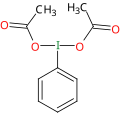The Willgerodt rearrangement or Willgerodt reaction is an organic reaction converting an aryl alkyl ketone, alkyne, or alkene to the corresponding amide...
5 KB (449 words) - 22:04, 14 May 2023
W X Y Z See also External links 1,2-Wittig rearrangement 1,3-Dipolar cycloaddition 2,3-Wittig rearrangement Acetalisation Acetoacetic ester condensation...
38 KB (3,423 words) - 21:05, 20 August 2024
in organic chemistry. This reagent was originally prepared by Conrad Willgerodt by reacting iodobenzene with a mixture of acetic acid and peracetic acid:...
12 KB (1,087 words) - 22:18, 16 July 2023
case of aryl–alkyl ketones, with sulfur and an amine give amides in the Willgerodt reaction With hydroxylamine to produce oximes With reducing agents to...
25 KB (2,945 words) - 17:13, 27 August 2024
Retrieved 23 May 2007. Schulenberg, J. W.; Archer, S. (1965). "The Chapman Rearrangement". Org. React. 14: 1–51. doi:10.1002/0471264180.or014.01. ISBN 978-0471264187...
24 KB (2,322 words) - 18:40, 26 August 2024
Oxidative rearrangements are generally easier to accomplish using hypervalent iodine reagents than other oxidizing agents. The Willgerodt-Kindler reaction...
10 KB (1,207 words) - 14:57, 28 August 2023