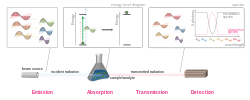
The emission spectrum of a chemical element or chemical compound is the spectrum of frequencies of electromagnetic radiation emitted due to electrons making...
23 KB (2,638 words) - 00:41, 10 July 2024
Absorption spectroscopy (redirect from Absorption spectrum)
Emission can occur at any frequency at which absorption can occur, and this allows the absorption lines to be determined from an emission spectrum. The...
22 KB (2,574 words) - 11:42, 9 April 2024
Black-body radiation (redirect from Black body spectrum)
an approximate black body with an emission spectrum peaked in the central, yellow-green part of the visible spectrum, but with significant power in the...
72 KB (9,264 words) - 10:06, 21 June 2024
absorption, and the emission monochromator scans the spectrum. For measuring excitation spectra, the wavelength passing through the emission filter or monochromator...
22 KB (2,841 words) - 16:12, 30 April 2024
Sunlight (redirect from Sun emission spectrum)
irradiance which can be focused on a surface using mirrors: 48.5 MW/m2. The spectrum of the Sun's solar radiation can be compared to that of a black body with...
52 KB (5,643 words) - 17:08, 25 June 2024
transitions from a lower to a higher energy state. The emission spectrum refers to the spectrum of radiation emitted by the compound due to electron transitions...
22 KB (2,729 words) - 18:37, 5 April 2024
Hydrogen spectral series (redirect from Hydrogen emission line)
The emission spectrum of atomic hydrogen has been divided into a number of spectral series, with wavelengths given by the Rydberg formula. These observed...
19 KB (1,910 words) - 00:34, 16 July 2024
released into the environment Emissions trading, a market-based approach to pollution control Emission spectrum, the spectrum of frequencies of electromagnetic...
2 KB (301 words) - 12:16, 3 October 2023
Beta decay (redirect from Beta emission)
electron emission. From 1920 to 1927, Charles Drummond Ellis (along with Chadwick and colleagues) further established that the beta decay spectrum is continuous...
58 KB (6,985 words) - 20:18, 21 June 2024
by lightning) is dominated by the emission lines of nitrogen, yielding the spectrum with primarily blue emission lines. The lines of neutral nitrogen...
12 KB (1,235 words) - 14:30, 12 July 2024
Spectral line (redirect from Emission line)
stronger region in an otherwise uniform and continuous spectrum. It may result from emission or absorption of light in a narrow frequency range, compared...
40 KB (2,361 words) - 13:47, 5 June 2024
Thermal radiation (redirect from Thermal emission)
dipole oscillation. At room temperature, most of the emission is in the infrared (IR) spectrum.: 73–86 Thermal radiation is one of the fundamental mechanisms...
56 KB (7,056 words) - 21:06, 7 July 2024
First, the emission spectrum of a broadband lamp is measured (this is called the "background spectrum"). Second, the emission spectrum of the same lamp...
15 KB (1,888 words) - 17:19, 20 March 2023
element in a sample. The wavelength of the atomic spectral line in the emission spectrum gives the identity of the element while the intensity of the emitted...
7 KB (861 words) - 18:52, 29 October 2023
found that roughly a third of them had the emission spectrum of a gas. The rest showed a continuous spectrum and were thus thought to consist of a mass...
21 KB (2,865 words) - 19:47, 21 July 2024
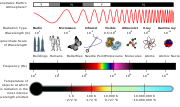
Electromagnetic radiation (redirect from Radiation emission)
absorption and emission spectrum. These bands correspond to the allowed energy levels in the atoms. Dark bands in the absorption spectrum are due to the...
80 KB (9,553 words) - 00:09, 26 July 2024
Spectroscopy (redirect from Emission spectrochemical analysis)
diffracted by a prism-like instrument displays either an absorption spectrum or an emission spectrum depending upon whether the element is being cooled or heated...
42 KB (4,648 words) - 07:22, 31 May 2024
"full-spectrum" so it cannot be measured. To compare "full-spectrum" sources requires direct comparison of spectral distribution. The emission spectrum of...
10 KB (1,205 words) - 09:10, 12 May 2023
loss and then stimulated emission can be used to boost an optical signal. Absorption (optics) Stimulated emission Emission spectrum Spectral line Atomic spectral...
21 KB (2,894 words) - 05:55, 24 June 2024
of characteristic spectral lines (monochromatic components of the emission spectrum) of chemical elements. However, they soon realized that the definition...
19 KB (2,041 words) - 01:13, 28 July 2024
atomic structure allowing a unique set of peaks on its electromagnetic emission spectrum (which is the main principle of spectroscopy). The peak positions...
12 KB (1,633 words) - 22:21, 4 May 2024
Balmer series (redirect from Balmer emission line)
The visible spectrum of light from hydrogen displays four wavelengths, 410 nm, 434 nm, 486 nm, and 656 nm, that correspond to emissions of photons by...
12 KB (1,321 words) - 06:57, 18 June 2024
energy radiated increases with temperature while the peak of the emission spectrum shifts to shorter wavelengths. The energy emitted at shorter wavelengths...
40 KB (4,204 words) - 21:07, 7 July 2024
Astronomical spectroscopy (redirect from Stellar spectrum)
a spectrum can be calibrated by observing the spectrum of emission lines of known wavelength from a gas-discharge lamp. The flux scale of a spectrum can...
47 KB (5,312 words) - 00:20, 10 July 2024
similar UV spectrum to deuterium, and have been used in UV spectroscopes. However, lamps using deuterium have a longer life span and an emissivity (intensity)...
6 KB (701 words) - 06:42, 24 November 2023
NGC 5508 is a LINER galaxy, i.e. a galaxy whose nucleus exhibits an emission spectrum characterized by broad lines of weakly ionized atoms. The Hubble distance...
4 KB (258 words) - 02:09, 26 July 2024
also reported in 2009 that the LCROSS probe revealed an ultraviolet emission spectrum consistent with hydroxyl presence. On 26 October 2020, NASA reported...
14 KB (1,505 words) - 11:58, 29 April 2024
For example, when the lens absorbs 350 nm light, the fluorescence emission spectrum is centered on 440 nm. In addition to the photopic and scotopic systems...
34 KB (3,902 words) - 02:16, 15 July 2024
Fluorophore (redirect from Emission peak)
and emission (for example, Absorption/Emission = 485 nm/517 nm) are the typical terms used to refer to a given fluorophore, but the whole spectrum may...
27 KB (2,024 words) - 06:28, 19 April 2024
is not applicable. The emission spectrum observed in flame test is also the basis of flame emission spectroscopy, atomic emission spectroscopy, and flame...
14 KB (974 words) - 06:20, 24 July 2024