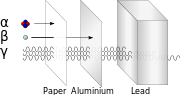
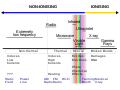
three ionization energies are defined as follows: 1st ionization energy is the energy that enables the reaction X ⟶ X+ + e− 2nd ionization energy is the...
51 KB (5,883 words) - 15:10, 14 May 2024
a singly, doubly, etc., charged ion. For ionization energies measured in the unit eV, see Ionization energies of the elements (data page). All data from...
25 KB (229 words) - 20:31, 29 June 2024
ionization rate is possible. Tunnel ionization is ionization due to quantum tunneling. In classical ionization, an electron must have enough energy to...
48 KB (6,714 words) - 23:23, 5 July 2024
column marked 1 is the first ionization energy to ionize the neutral atom, the column marked 2 is the second ionization energy to remove a second electron...
63 KB (870 words) - 17:41, 8 January 2024
therefore the bulk of the ionization effects are due to secondary ionization. Even though photons are electrically neutral, they can ionize atoms indirectly through...
60 KB (6,553 words) - 05:22, 3 June 2024
Periodic trends (section Ionization energy)
the nucleus. It is also referred to as ionization potential. The first ionization energy is the amount of energy that is required to remove the first electron...
15 KB (1,607 words) - 23:17, 5 July 2024
Ion (section Ionization potential)
electric charge is called the ionization potential, or ionization energy. The nth ionization energy of an atom is the energy required to detach its nth electron...
30 KB (3,020 words) - 20:58, 7 May 2024
Radiation (section Ionizing radiation)
enough heat to raise temperatures to ionization energies. These reactions occur at far higher energies than with ionization radiation, which requires only single...
47 KB (6,147 words) - 21:47, 17 July 2024
process are ionized. Thermal ionization is used to make simple ion sources, for mass spectrometry and for generating ion beams. Thermal ionization has seen...
5 KB (652 words) - 00:40, 1 September 2023
Stopping power (particle radiation) (redirect from Non-ionizing energy loss)
electron) requires a fixed amount of energy (for example, 33.97 eV in dry air: 305 ), the number of ionizations per path length is proportional to the...
28 KB (3,729 words) - 09:09, 4 November 2023
Electron ionization (EI, formerly known as electron impact ionization and electron bombardment ionization) is an ionization method in which energetic electrons...
31 KB (4,003 words) - 06:11, 3 July 2024
Ion source (redirect from Atmospheric pressure ionization)
Electron ionization is widely used in mass spectrometry, particularly for organic molecules. The gas phase reaction producing electron ionization is M +...
58 KB (7,140 words) - 08:46, 21 February 2024
(2006–) "Ionization energy". doi:10.1351/goldbook.I03199 "Binding Energy". Nuclear Power. Retrieved 16 May 2015. Bodansky, David (2005). Nuclear Energy: Principles...
14 KB (1,342 words) - 19:25, 29 June 2024
In physics, the Saha ionization equation is an expression that relates the ionization state of a gas in thermal equilibrium to the temperature and pressure...
13 KB (1,904 words) - 21:06, 8 July 2024
first two ionization energies for europium, 1632 kJ·mol−1 can be compared with that of barium 1468.1 kJ·mol−1 and europium's third ionization energy is the...
107 KB (10,251 words) - 17:33, 29 June 2024
Rydberg state (section Energy of Rydberg states)
electronically excited states with energies that follow the Rydberg formula as they converge on an ionic state with an ionization energy. Although the Rydberg formula...
6 KB (653 words) - 05:59, 6 May 2024
thermal-ionization if it deposits enough heat to raise temperatures to ionization energies. These reactions occur at far higher energies than with ionizing radiation...
22 KB (2,516 words) - 01:45, 10 July 2024
Plasma (physics) (redirect from Ionized gas)
a plasma. The degree of plasma ionization is determined by the electron temperature relative to the ionization energy (and more weakly by the density)...
62 KB (6,398 words) - 23:43, 27 June 2024
but this generally requires low ionization energies. Instead, because of its small size and high ionization energies, the basic structural unit of boron...
248 KB (28,088 words) - 14:32, 6 July 2024
electronegativity of an atom is strongly correlated with the first ionization energy. The electronegativity is slightly negatively correlated (for smaller...
36 KB (4,507 words) - 20:10, 13 May 2024
Paschen's law (section Impact ionization)
which can be accelerated to trigger impact ionization. Such so-called seed electrons can be created by ionization by natural radioactivity or cosmic rays...
22 KB (2,788 words) - 18:38, 24 June 2024
first ionization energy is −24.587387936(25) eV. This value was measured experimentally. The theoretic value of Helium atom's second ionization energy is...
35 KB (6,005 words) - 00:34, 16 July 2024
Ditungsten tetra(hpp) (redirect from Lowest ionization energy compound)
acetate. The molecule is of research interest because it has the lowest ionization energy (3.51 eV) of all stable chemical elements or chemical compounds as...
4 KB (338 words) - 18:29, 12 July 2024
meaningful form for the ionization rate above can be obtained by noting that the Bohr radius and hydrogen atom ionization energy are given by a 0 = 4 π...
24 KB (3,863 words) - 20:42, 5 June 2024
orbital approximation). Ionization energies calculated this way are in qualitative agreement with experiment – the first ionization energy of small molecules...
24 KB (2,894 words) - 05:33, 25 October 2023
Hence, its first ionization energy would be smaller than thorium's (Th: 6.3 eV; element 122: 5.6 eV) because of the greater ease of ionizing unbibium's 8p1/2...
148 KB (15,124 words) - 15:17, 17 July 2024
predicted to have a lower ionization energy of 4.45 eV, so that the alkali metal in period 8 would not have the lowest ionization energy in the period, as is...
42 KB (8,085 words) - 09:53, 19 July 2024
highly ionized regions (H II regions and the Warm Ionised Medium). Carbon has a lower ionization energy than hydrogen, and so singly-ionized carbon atoms...
51 KB (5,769 words) - 19:59, 11 July 2024
element in group 1 of the periodic table. They have a similar first ionization energy, which allows for each atom to give up its sole outer electron. It...
94 KB (10,896 words) - 21:10, 19 July 2024
conductive region is less sharp, the ionization may not extend past this local region. Outside this region of ionization and conductivity, the charged particles...
24 KB (3,073 words) - 10:20, 22 June 2024