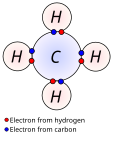
In chemistry, valence bond (VB) theory is one of the two basic theories, along with molecular orbital (MO) theory, that were developed to use the methods...
12 KB (1,576 words) - 07:10, 29 April 2024
Modern valence bond theory is the application of valence bond theory (VBT) with computer programs that are competitive in accuracy and economy, with programs...
20 KB (2,703 words) - 07:52, 11 June 2024
theory (DFT) or Hartree–Fock (HF) models to the Schrödinger equation. Molecular orbital theory and valence bond theory are the foundational theories of...
22 KB (2,956 words) - 17:38, 23 September 2024
Modern valence bond theory is often seen as an extension of the Coulson–Fischer method. Coulson–Fischer theory is an extension of modern valence bond...
4 KB (613 words) - 12:57, 4 November 2023
generalized valence bond (GVB) is a method in valence bond theory that uses flexible orbitals in the general way used by modern valence bond theory. The method...
3 KB (325 words) - 16:21, 23 February 2023
single covalent bond, a shared pair forms with both atoms in the bond each contributing one valence electron. The presence of valence electrons can determine...
24 KB (2,333 words) - 22:12, 20 May 2024
share "valence", such as is discussed in valence bond theory. In the molecule H 2, the hydrogen atoms share the two electrons via covalent bonding. Covalency...
28 KB (3,673 words) - 00:25, 28 September 2024
causes of valence led to the modern theories of chemical bonding, including the cubical atom (1902), Lewis structures (1916), valence bond theory (1927)...
40 KB (2,914 words) - 06:38, 15 August 2024
unified bonding model. Instead, several traditional and advanced bonding models such as simple Lewis and VSEPR structure, valence bond theory, molecular...
15 KB (1,822 words) - 11:34, 13 March 2023
polarity of bonds. The octet rule and VSEPR theory are examples. More sophisticated theories are valence bond theory, which includes orbital hybridization and...
40 KB (4,872 words) - 13:33, 22 September 2024
Valence shell electron pair repulsion (VSEPR) theory (/ˈvɛspər, vəˈsɛpər/ VESP-ər,: 410 və-SEP-ər) is a model used in chemistry to predict the geometry...
45 KB (4,038 words) - 14:24, 26 September 2024
Orbital hybridisation (redirect from Sp² bond)
bonds in valence bond theory. For example, in a carbon atom which forms four single bonds, the valence-shell s orbital combines with three valence-shell...
33 KB (3,169 words) - 13:02, 22 September 2024
other modern valence bond theory methods. Molecular orbital theory Basis set (chemistry) Weinhold, Frank; Landis, Clark R. (2001). "Natural Bond Orbitals...
5 KB (548 words) - 21:13, 16 October 2022
Electronic band structure (redirect from Band theory)
called the valence band. The name "valence band" was coined by analogy to chemistry, since in semiconductors (and insulators) the valence band is built...
37 KB (4,835 words) - 14:38, 19 August 2024
Hartree–Fock method (redirect from Hartree-Fock theory)
scheme is one such hybrid functional method. Another option is to use modern valence bond methods. For a list of software packages known to handle Hartree–Fock...
31 KB (4,729 words) - 12:30, 5 August 2024
Hypervalent molecule (redirect from Hypervalent bonding)
the bonding of hypervalent species, the energetic contribution of an expanded octet structure is also not null. In this modern valence bond theory study...
39 KB (4,486 words) - 20:28, 22 July 2024
interacts with the valence electrons. The use of an effective interaction, a pseudopotential, that approximates the potential felt by the valence electrons, was...
79 KB (10,545 words) - 09:18, 9 September 2024
Valence bond (VB) computer programs for modern valence bond calculations:-[1] CRUNCH, by Gordon A. Gallup and his group. GAMESS (UK), includes calculation...
3 KB (395 words) - 12:21, 3 September 2024
In condensed matter physics, the resonating valence bond theory (RVB) is a theoretical model that attempts to describe high-temperature superconductivity...
5 KB (597 words) - 10:23, 14 August 2023
been proposed. Current VB approaches are:- Generalized valence bond (GVB) Modern valence bond theory (MVBT) A method that avoids making the variational overestimation...
21 KB (2,524 words) - 08:10, 7 September 2024
Octet rule (redirect from Lewis-Langmuir theory)
thumb that reflects the theory that main-group elements tend to bond in such a way that each atom has eight electrons in its valence shell, giving it the...
22 KB (2,869 words) - 19:16, 11 May 2024
multi-reference, or valence-universal multi-reference coupled cluster approaches. The wavefunction of the coupled-cluster theory is written as an exponential...
28 KB (4,353 words) - 18:46, 19 July 2024
systems, this was the Hückel method proposed by Erich Hückel. For all valence electron systems, the extended Hückel method was proposed by Roald Hoffmann...
12 KB (1,419 words) - 06:03, 22 August 2024
the same sort of perturbation-theory analysis as above. For degenerate or nearly degenerate bands, in particular the valence bands in certain materials such...
12 KB (1,975 words) - 11:29, 25 March 2024
Resonance (chemistry) (redirect from Theory of Resonance)
(or hybrid structure) in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one...
42 KB (5,094 words) - 06:04, 25 June 2024
functional theory are not adequate (e.g., for molecular ground states which are quasi-degenerate with low-lying excited states or in bond-breaking situations)...
8 KB (1,120 words) - 14:58, 16 September 2024
model are the interatomic matrix elements, which would simply be called the bond energies by a chemist. In general there are a number of atomic energy levels...
34 KB (7,350 words) - 03:42, 26 August 2024
stretched bonds. Bond order is also an index of bond strength and is also used extensively in valence bond theory. bond order = number of bonding electrons...
9 KB (1,260 words) - 05:21, 12 February 2023
Møller–Plesset perturbation theory (MP) is one of several quantum chemistry post-Hartree–Fock ab initio methods in the field of computational chemistry...
23 KB (2,870 words) - 20:17, 25 November 2023
of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms. A modern conceptualization...
36 KB (4,474 words) - 14:38, 25 April 2024