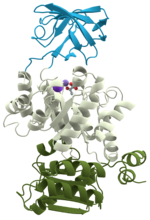
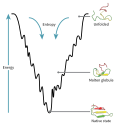Protein folding is the physical process by which a protein, after synthesis by a ribosome as a linear chain of amino acids, changes from an unstable random...
76 KB (8,728 words) - 11:37, 6 November 2024
convey any steric information for protein folding, and instead assist in protein folding by binding to and stabilizing folding intermediates until the polypeptide...
29 KB (3,499 words) - 07:16, 20 February 2024
protein folding into a specific 3D structure that determines its activity. A linear chain of amino acid residues is called a polypeptide. A protein contains...
103 KB (11,472 words) - 16:37, 31 October 2024
comprehensive views of the folding process. Protein folding does not occur in one step. Instead, proteins spend most of their folding time, nearly 96% in some...
154 KB (14,621 words) - 06:37, 11 November 2024
answer to the protein prediction problem would still leave questions about the protein folding problem—understanding in detail how the folding process actually...
63 KB (5,691 words) - 20:37, 8 November 2024
predict protein folding and thus protein structure, for example, Itasser, and AlphaFold. AlphaFold was one of the first AIs to predict protein structures....
71 KB (9,035 words) - 04:09, 27 October 2024
Huntington's disease. The term protein folding incorporates all the processes involved in the production of a protein after the nascent polypeptides have...
31 KB (3,592 words) - 02:08, 11 November 2024
protein structure that could fold autonomously. In the past domains have been described as units of: compact structure function and evolution folding...
71 KB (8,443 words) - 19:00, 15 August 2024
protein folding by increasing solubility and mediates the protein binding to protein chaperones. Chaperones are proteins responsible for folding and maintaining...
40 KB (4,342 words) - 13:04, 6 November 2024
The folding funnel hypothesis is a specific version of the energy landscape theory of protein folding, which assumes that a protein's native state corresponds...
19 KB (2,446 words) - 20:44, 26 September 2024
perceived importance Paper folding, or origami, the art of folding paper Protein folding, the physical process by which a polypeptide folds into its characteristic...
3 KB (444 words) - 21:15, 7 July 2024
perform chaperone functions by stabilizing new proteins to ensure correct folding or by helping to refold proteins that were damaged by the cell stress. This...
49 KB (5,533 words) - 08:12, 2 November 2024
of protein folding is currently being studied. Even in the protein's denatured state, it can be folded into the correct structure. Globular proteins seem...
6 KB (746 words) - 12:06, 14 October 2024
identified as a peptide, rather than a protein. To be able to perform their biological function, proteins fold into one or more specific spatial conformations...
37 KB (4,208 words) - 04:07, 27 October 2024
Denaturation (biochemistry) (redirect from Denatured protein)
must be folded into the native shape to function. However, hydrogen bonds and cofactor-protein binding, which play a crucial role in folding, are rather...
30 KB (3,182 words) - 02:16, 27 October 2024
transcription, translation, post translational modifications, and protein folding. Proteins are made from amino acids. In humans, some amino acids can be...
27 KB (2,828 words) - 23:11, 3 November 2024
Hydrophobic effect (section Folding of macromolecules)
membrane and vesicle formation, protein folding, insertion of membrane proteins into the nonpolar lipid environment and protein-small molecule associations...
13 KB (1,505 words) - 13:33, 21 August 2024
molecular biology, protein fold classes are broad categories of protein tertiary structure topology. They describe groups of proteins that share similar...
9 KB (961 words) - 21:22, 29 July 2024
beta-barrel transmembrane proteins have simplest up-and-down topology, which may reflect their common evolutionary origin and similar folding mechanism.[citation...
21 KB (2,262 words) - 15:51, 18 July 2024
Hsp90 (redirect from Heat shock protein 90)
shock protein 90) is a chaperone protein that assists other proteins to fold properly, stabilizes proteins against heat stress, and aids in protein degradation...
51 KB (5,551 words) - 15:05, 1 October 2024
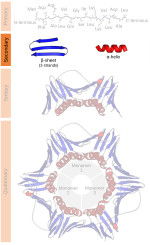
helix and alpha sheet are rare in native state proteins but are often hypothesized as important protein folding intermediates. Tight turns and loose, flexible...
28 KB (3,076 words) - 03:33, 27 October 2024
fully folded state, and therefore the enzyme acts to catalyze protein folding. Protein disulfide-isomerase has two catalytic thioredoxin-like domains...
19 KB (1,936 words) - 16:09, 10 July 2024
Levinthal's paradox (category Protein structure)
proteins fold by subunits (modules) of the size of 25–30 amino acids. Chaperone – proteins that assist other proteins in folding or unfolding Folding...
8 KB (919 words) - 13:28, 9 October 2024
Hence, proteins may be classified by the structures they hold. Databases of proteins which use such a classification include SCOP and CATH. Folding kinetics...
15 KB (1,603 words) - 16:54, 15 June 2024
Molecular biophysics (redirect from Protein chemistry)
nanorobots are far beyond current capabilities. Protein folding is the physical process by which a protein chain acquires its native 3-dimensional structure...
23 KB (2,450 words) - 14:34, 16 May 2024
Google DeepMind (section Protein folding)
advances in the problem of protein folding with AlphaFold. In July 2022, it was announced that over 200 million predicted protein structures, representing...
82 KB (7,998 words) - 15:26, 28 October 2024
GroEL (redirect from Groel protein)
is a protein which belongs to the chaperonin family of molecular chaperones, and is found in many bacteria. It is required for the proper folding of many...
42 KB (5,440 words) - 05:47, 10 June 2024
experiment, one can deduce that protein folding must not be a completely random process and that information necessary for folding must be encoded within the...
23 KB (2,842 words) - 02:01, 27 October 2024
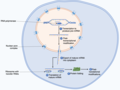
Endoplasmic reticulum (section Protein transport)
the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic...
34 KB (3,710 words) - 13:38, 2 November 2024
Hsp70 (redirect from Heat-shock protein 70)
localized Hsp70s are an important part of the cell's machinery for protein folding, performing chaperoning functions, and helping to protect cells from...
50 KB (5,687 words) - 08:38, 2 November 2024