Copper(II) nitrate describes any member of the family of inorganic compounds with the formula Cu(NO3)2(H2O)x. The hydrates are blue solids. Anhydrous copper...
15 KB (1,333 words) - 14:55, 4 October 2024
(II) salts) are often blue to green in color, rather than the orange color copper is known for. Despite being considered a semi-noble metal, copper is...
8 KB (101 words) - 09:36, 4 August 2024
Copper(I) nitrate is a proposed inorganic compound with formula of CuNO3. It has not been characterized by X-ray crystallography. It is the focus of one...
3 KB (215 words) - 20:34, 12 October 2024
copper(II) oxide is conveniently prepared by pyrolysis of copper(II) nitrate or basic copper(II) carbonate: 2 Cu(NO3)2 → 2 CuO + 4 NO2 + O2 (180°C) Cu2(OH)2CO3...
10 KB (829 words) - 15:20, 14 September 2024
crystallized. In one the monodentate nitrate ligands are trans while in the other they are cis. Nickel(II) nitrate is primarily used in electrotyping and...
6 KB (391 words) - 01:29, 8 October 2024
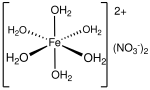
Iron(II) nitrate is the nitrate salt of iron(II). It is commonly encountered as the green hexahydrate, Fe(NO3)2·6H2O, which is a metal aquo complex, however...
8 KB (733 words) - 00:18, 23 October 2024
Zinc nitrate is an inorganic chemical compound with the formula Zn(NO3)2. This colorless, crystalline salt is highly deliquescent. It is typically encountered...
5 KB (394 words) - 13:43, 19 June 2024
triphenylphosphine) Given nitrate's low basicity, the tendency of metal nitrate complexes toward hydrolysis is expected. Thus copper(II) nitrate readily dissociates...
11 KB (1,122 words) - 22:26, 27 August 2024
following the element name. For example, Cu(NO3)2 is copper(II) nitrate, because the charge of two nitrate ions (NO− 3) is 2 × −1 = −2, and since the net charge...
13 KB (1,468 words) - 15:58, 27 September 2024
and nitric acid produce copper(II) sulfate and copper(II) nitrate, respectively. In terms of their coordination spheres, copper centres are 2-coordinated...
12 KB (1,049 words) - 17:26, 4 October 2024
non-explosive products, these being nitrogen, nitrogen oxides and copper(II) nitrate. Lide, David R. (1998), Handbook of Chemistry and Physics (87 ed.)...
3 KB (127 words) - 15:00, 29 October 2023
acetate, copper(II) nitrate, and copper(II) carbonate. Copper(II) sulfate forms a blue crystalline pentahydrate, the most familiar copper compound in the...
122 KB (13,910 words) - 18:45, 24 October 2024
hydroxide – Cu(OH)2 Copper(II) nitrate – Cu(NO3)2 Copper(II) oxide – CuO Copper(II) sulfate – CuSO4 Copper(II) sulfide – CuS Copper oxychloride – H3ClCu2O3...
119 KB (8,735 words) - 17:31, 25 October 2024
active copper chromite catalyst which includes barium in its structure can be prepared from a solution containing barium nitrate, copper(II) nitrate, and...
10 KB (991 words) - 01:56, 23 October 2024
A nitrate test is a chemical test used to determine the presence of nitrate ion in solution. Testing for the presence of nitrate via wet chemistry is generally...
6 KB (723 words) - 17:34, 4 September 2024
acetate, copper(II) nitrate, and copper(II) carbonate. Copper(II) sulfate forms a blue crystalline pentahydrate, the most familiar copper compound in the...
14 KB (1,500 words) - 07:17, 30 August 2024
diazonium salt. In the presence of copper catalyst, such as copper(I) oxide, and an excess of copper(II) nitrate, this reaction takes place readily at...
16 KB (1,738 words) - 18:24, 22 October 2024
Nitrate chlorides are mixed anion compounds that contain both nitrate (NO3−) and chloride (Cl−) ions. Various compounds are known, including amino acid...
63 KB (3,736 words) - 12:04, 2 November 2024
Nitric acid (redirect from Hydrogen nitrate)
industry) with either sulfuric acid or magnesium nitrate. Alternatively, thermal decomposition of copper(II) nitrate gives nitrogen dioxide and oxygen gases;...
46 KB (5,169 words) - 06:52, 18 October 2024
bidentate nitrate ligands. The Ag-O distances range from 2.384 to 2.702 Å. A typical reaction with silver nitrate is to suspend a rod of copper in a solution...
24 KB (2,316 words) - 01:04, 29 October 2024
Salser (1973). "Mass spectra of covalent inorganic nitrates: copper(II) nitrate and titanium(IV) nitrate". Journal of Inorganic and Nuclear Chemistry. 35...
9 KB (764 words) - 00:49, 10 December 2021
needed] Mercury(II) nitrate and potassium nitrate in water solution produce the salt tripotassium tetranitratomercurate(II) nitrate K3[Hg(NO2)4]NO3....
12 KB (831 words) - 00:52, 17 May 2024
calculations of an oxalato-bridged copper(II) complex: μ-oxalato-bis[(2,2'-bipyridine) hydrate copper(II) nitrate]". Journal of the Iranian Chemical Society...
38 KB (2,143 words) - 13:35, 5 March 2024
Copper(II) iodate Cu(IO3)2·2H2O 0.109 Copper(II) nitrate Cu(NO3)2 83.5 100 125 156 163 182 208 222 247 Copper oxalate CuC2O4·2H2O 2.1627×10−10 Copper(II)...
84 KB (196 words) - 10:05, 30 October 2024
aqueous copper(II) nitrate (Cu(NO3)2) and solid silver. How much silver is produced if 16.00 grams of Cu is added to the solution of excess silver nitrate? The...
35 KB (5,278 words) - 05:19, 3 November 2024
copper(II) arsenite 10290–12–7 CuI copper(I) iodide 7681–65–4 CuMoO4 copper(II) molybdate 13767–34–5 Cu(NO3)2 copper(II) nitrate 3251–23–8 CuN3 copper(I)...
139 KB (120 words) - 17:07, 15 July 2024
copper(I) iodate Cu(IO3)2 copper(II) iodate 13454-89-2 CuMoO4 copper(II) orthomolybdate Cu(NO3)2 copper(II) nitrate 3251-23-8 Cu(NO3)2 · 3H2O copper(II)...
183 KB (107 words) - 22:11, 6 September 2024
tetrafluoroborate, and nitrate are known. The cation was first reported in 1923 with a nitrate anion as a byproduct of the reduction of silver nitrate with a suspension...
5 KB (512 words) - 21:51, 25 July 2024
Nitrate is a polyatomic ion with the chemical formula NO− 3. Salts containing this ion are called nitrates. Nitrates are common components of fertilizers...
32 KB (3,498 words) - 16:23, 4 September 2024
Thermite (section Copper thermite)
chromium(III) oxide, manganese(IV) oxide, iron(III) oxide, iron(II,III) oxide, copper(II) oxide, and lead(II,IV) oxide. In a thermochemical survey comprising twenty-five...
50 KB (5,823 words) - 21:53, 28 October 2024