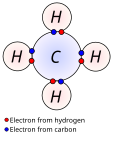
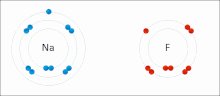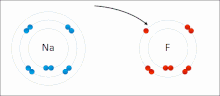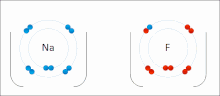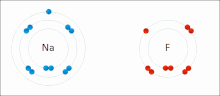
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure)...
60 KB (6,206 words) - 06:35, 26 December 2024
This page shows the electron configurations of the neutral gaseous atoms in their ground states. For each atom the subshells are given first in concise...
58 KB (1,187 words) - 18:52, 1 December 2024
Periodic table (section Electron configuration table)
(period) is started when a new electron shell has its first electron. Columns (groups) are determined by the electron configuration of the atom; elements with...
251 KB (27,112 words) - 18:30, 24 December 2024
dependent upon its electronic configuration. For a main-group element, a valence electron can exist only in the outermost electron shell; for a transition metal...
24 KB (2,333 words) - 15:26, 27 November 2024
Configurations of elements 109 and above are not available. Predictions from reliable sources have been used for these elements. Grayed out electron numbers...
3 KB (303 words) - 20:18, 18 May 2023
Ionization energy (redirect from Electron binding energy)
determining their respective electron configuration. Nuclear charge: If the nuclear charge (atomic number) is greater, the electrons are held more tightly by...
52 KB (5,903 words) - 19:02, 21 November 2024
being able to hold up to 2(n2) electrons. For an explanation of why electrons exist in these shells, see electron configuration. Each shell consists of one...
28 KB (2,778 words) - 04:23, 10 December 2024
Aufbau principle (redirect from Principles in distribution of electrons)
the 1s subshell has 2 electrons, the 2s subshell has 2 electrons, the 2p subshell has 6 electrons, and so on. The configuration is often abbreviated by...
28 KB (3,091 words) - 16:31, 10 December 2024
topological space Configuration space (physics), in classical mechanics, the vector space formed by the parameters of a system Electron configuration, the distribution...
2 KB (257 words) - 16:23, 1 June 2023
Lewis structure (redirect from Electron Dot Structure)
losing, or sharing electrons until they have achieved a valence shell electron configuration with a full octet of (8) electrons, hydrogen (H) can only...
16 KB (2,138 words) - 02:32, 26 November 2024
Ion (redirect from Free floating electrons)
few electrons short of a stable configuration. As such, they have the tendency to gain more electrons in order to achieve a stable configuration. This...
31 KB (3,178 words) - 08:15, 23 November 2024
nonmetal) with greater electron affinity accepts one or more electrons to attain a stable electron configuration, and after accepting electrons an atom becomes...
18 KB (2,338 words) - 02:36, 8 February 2024
The d electron count or number of d electrons is a chemistry formalism used to describe the electron configuration of the valence electrons of a transition...
14 KB (1,701 words) - 03:27, 4 October 2023
Covalent bond (redirect from One-electron bond)
chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs...
28 KB (3,673 words) - 16:31, 3 December 2024
Transition metal (section Electronic configuration)
that n = 4, the first 18 electrons have the same configuration of Ar at the end of period 3, and the overall configuration is [Ar]3d24s2. The period...
40 KB (4,504 words) - 13:03, 5 December 2024
represents an actual value of a physical quantity. For a given electron configuration of an atom, its state depends also on its total angular momentum...
57 KB (5,847 words) - 15:16, 23 October 2024
Atomic orbital (redirect from Electron cloud)
matter. In this model, the electron cloud of an atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler...
84 KB (10,942 words) - 01:33, 19 November 2024
element 164 with a 7d109s0 electron configuration shows clear analogies with palladium with its 4d105s0 electron configuration. The noble metals of this...
150 KB (15,241 words) - 21:53, 2 January 2025
repulsion. B and C correspond with individual d-electron repulsions. A is constant among d-electron configuration, and it is not necessary for calculating relative...
21 KB (2,288 words) - 18:18, 4 November 2024
Noble gas (section Electron configuration)
other chemical substances, results from their electron configuration: their outer shell of valence electrons is "full", giving them little tendency to participate...
95 KB (9,690 words) - 19:39, 19 December 2024
Nitrene (section Electron configuration)
non-bonded electrons as a lone pair in an sp orbital and the other two occupying a degenerate pair of p orbitals. The electron configuration is consistent...
17 KB (1,870 words) - 03:49, 30 December 2024
for more details.) For an explanation of why electrons exist in these shells see electron configuration. If the potential energy is set to zero at infinite...
22 KB (2,832 words) - 12:10, 3 January 2025
The rule is based on the fact that the valence orbitals in the electron configuration of transition metals consist of five (n−1)d orbitals, one ns orbital...
17 KB (1,923 words) - 02:05, 19 June 2024
Nickel (section Electron configuration dispute)
some disagreement on which configuration has the lower energy. Chemistry textbooks quote nickel's electron configuration as [Ar] 4s2 3d8, also written...
89 KB (9,836 words) - 11:56, 24 December 2024
sometimes requiring identity of the total electron count and with it the entire electronic configuration. More usually, definitions are broader, and...
5 KB (436 words) - 14:21, 10 March 2024
or more open electronic shells. The rule states that for a given electron configuration, the lowest energy term is the one with the greatest value of spin...
6 KB (787 words) - 21:15, 18 February 2024
an electron configuration closely similar to that of a free atom in an external field, except that the outer parts of the electron configurations surrounding...
23 KB (3,018 words) - 12:43, 22 October 2024
Octet rule (section Three-electron bonds)
such a way that each atom has eight electrons in its valence shell, giving it the same electronic configuration as a noble gas. The rule is especially...
22 KB (2,870 words) - 20:18, 28 October 2024
referred to simply as Hund's Rule. The three rules are: For a given electron configuration, the term with maximum multiplicity has the lowest energy. The multiplicity...
12 KB (1,584 words) - 19:33, 4 December 2024
orbital, describing the behaviour of an electron in a molecule Electron configuration, the arrangement of electrons in structures such as atoms or molecules...
472 bytes (94 words) - 08:06, 27 October 2017