from trigonal to tetrahedral. Tetrahedral intermediates result from nucleophilic addition to a carbonyl group. The stability of tetrahedral intermediate depends...
18 KB (2,329 words) - 20:47, 22 July 2024
Nickel tetracarbonyl (redirect from Nickel(II) carbonyl)
Nickel carbonyl (IUPAC name: tetracarbonylnickel) is a nickel(0) organometallic compound with the formula Ni(CO)4. This colorless liquid is the principal...
18 KB (1,634 words) - 10:55, 15 October 2024
Borane carbonyl is the inorganic compound with the formula H3BCO. This colorless gas is the adduct of borane and carbon monoxide. It is usually prepared...
6 KB (532 words) - 13:50, 22 October 2023
attached to a normal flask. Cinnamic acid Dibenzylideneacetone Tetrahedral carbonyl addition compound Lassar-Cohn (1906). Arbeitsmethoden für organisch-chemische...
7 KB (778 words) - 08:27, 12 November 2024
Ene reaction (redirect from Carbonyl-ene reaction)
In the case of directed carbonyl-ene reactions, high levels of regio- and stereo-selectivity have been observed upon addition of a Lewis acid, which can...
27 KB (3,345 words) - 05:39, 27 October 2024
Acyl group (section Compounds)
(benzyl). Acyl compounds react with nucleophiles via an addition mechanism: the nucleophile attacks the carbonyl carbon, forming a tetrahedral intermediate...
18 KB (1,913 words) - 21:16, 5 November 2024
Grignard reagent (redirect from Grignard compound)
before the addition of the carbonyl compound. The solution becomes cloudy as the Grignard reagent precipitates out. A solution of carbonyl compound is added...
24 KB (2,752 words) - 01:37, 23 October 2024
electrons will adopt tetrahedral geometry with six M-M bonds. Tetrahedral clusters is classified as nido clusters. By addition of 2e, the 60e cluster...
2 KB (208 words) - 23:36, 26 April 2023
classified following some general principles. Tetrahedral SiO units are common to almost all these compounds, either as discrete structures, or combined...
42 KB (5,607 words) - 22:19, 30 May 2024
Organolithium reagent (redirect from Organolithium compound)
the lithium ion between the N-methoxy oxygen and the carbonyl oxygen, which forms a tetrahedral intermediate that collapses upon acidic work up. Organolithium...
55 KB (5,971 words) - 20:35, 22 July 2024
Metal carbonyls are coordination complexes of transition metals with carbon monoxide ligands. Metal carbonyls are useful in organic synthesis and as catalysts...
70 KB (7,931 words) - 06:04, 29 May 2024
Urea (redirect from Carbonyl diamide)
carbonic acid), is an organic compound with chemical formula CO(NH2)2. This amide has two amino groups (–NH2) joined by a carbonyl functional group (–C(=O)–)...
61 KB (7,066 words) - 14:31, 30 October 2024
Ester (redirect from Ester compound)
condensation, an enolate of one ester (1) will attack the carbonyl group of another ester (2) to give tetrahedral intermediate 3. The intermediate collapses, forcing...
46 KB (4,846 words) - 04:43, 20 November 2024
oxidation starts with (1) nucleophilic addition of a hydroperoxide ion to the carbonyl carbon, forming a (2) tetrahedral intermediate. The intermediate collapses...
15 KB (1,516 words) - 13:25, 26 August 2024
variety of compounds, usually with oxidation states +1 and +2, which are often called cuprous and cupric, respectively. Copper compounds, whether organic...
14 KB (1,500 words) - 07:17, 30 August 2024
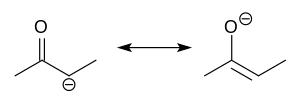
deprotonation of carbonyl (RR'C=O) compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds. Enolate anions are...
21 KB (2,467 words) - 22:07, 14 August 2024
002. The carboxylic group is the most acidic in organic compounds. As with all carbonyl compounds, the protons on the α-carbon are labile due to keto–enol...
26 KB (2,619 words) - 13:56, 13 October 2024
Organozinc chemistry (redirect from Organozinc compound)
Ryoji; Kitamura, Masato (1991). "Enantioselective Addition of Organometallic Reagents to Carbonyl Compounds: Chirality Transfer, Multiplication, and Amplification"...
33 KB (3,394 words) - 06:05, 9 December 2023
nickel arrangements can be square planar, or tetrahedral. Five coordinated nickel is rarer. Some nickel compounds are ferromagnetic at sufficiently low temperatures...
55 KB (6,147 words) - 19:11, 22 October 2024
β-unsaturated compounds. The Mukaiyama aldol reaction and the Sakurai reaction refer to the addition of silyl enol ethers and allylsilanes to carbonyl compounds, respectively...
45 KB (5,026 words) - 04:16, 15 October 2024
Acyl chloride (section Oxidative addition)
catalysis mechanism. The amine attacks the carbonyl bond and presumably first forms a transient tetrahedral intermediate, then forms a quaternary acylammonium...
20 KB (2,351 words) - 22:26, 31 October 2024
Acetal (section Related compounds)
compared to the analogous carbonyl compounds. The central carbon atom has four bonds to it, and is therefore saturated and has tetrahedral geometry. The term...
9 KB (1,122 words) - 00:44, 4 November 2024
Organotitanium chemistry (redirect from Organotitanium compound)
tetraalkyltitanium compounds however are not typically isolable, owing to the large size of titanium and the electron-deficient nature of its tetrahedral complexes...
18 KB (1,866 words) - 09:45, 4 November 2023
introduce a formyl group. Here, phenyllithium 1 attacks the carbonyl group of DMF 2, giving tetrahedral intermediate 3. Because the dimethylamide anion is a...
24 KB (2,311 words) - 11:52, 8 November 2024
Organocopper chemistry (redirect from Organocopper compound)
is polymeric. In contrast to classical metal carbonyls, pi-backbonding is not strong in these compounds. Alkenes bind to copper(I), although again generally...
23 KB (2,441 words) - 02:00, 1 August 2024
Inorganic chemistry (section Coordination compounds)
[Co(NH3)6]3+, TiCl4(THF)2. Coordination compounds show a rich diversity of structures, varying from tetrahedral for titanium (e.g., TiCl4) to square planar...
29 KB (3,220 words) - 00:35, 17 November 2024
as the near-tetrahedral diphosphine and diarsine complexes [Ag(L–L)2]+. Under standard conditions, silver does not form simple carbonyls, due to the weakness...
18 KB (2,206 words) - 02:26, 1 November 2023
Bismuth organometallic chemistry (category Bismuth compounds)
stereoisomers convert to a common spiro [4.3] cluster compound. Adding to the transition metal-bismuth carbonyl clusters, the dibismuth clusters with transition...
16 KB (1,573 words) - 20:14, 23 April 2024
Iron pentacarbonyl (category Iron carbonyl complexes)
Iron pentacarbonyl, also known as iron carbonyl, is the compound with formula Fe(CO)5. Under standard conditions Fe(CO)5 is a free-flowing, straw-colored...
17 KB (1,657 words) - 15:14, 21 November 2024