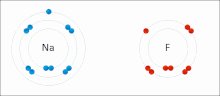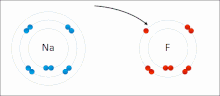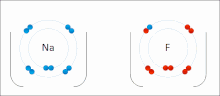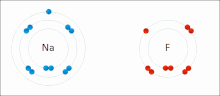
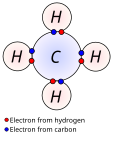
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms. These electron pairs are known as shared...
28 KB (3,673 words) - 05:14, 23 November 2024
coordinate covalent bond, also known as a dative bond, dipolar bond, or coordinate bond is a kind of two-center, two-electron covalent bond in which the...
10 KB (1,313 words) - 13:10, 17 August 2024
covalent bonds. Also, the melting points of such covalent polymers and networks increase greatly. In a simplified view of an ionic bond, the bonding electron...
40 KB (4,872 words) - 13:33, 22 September 2024
Chemical polarity (redirect from Polar covalent bond)
usually applied to covalent bonds, that is, bonds where the polarity is not complete. To determine the polarity of a covalent bond using numerical means...
24 KB (2,751 words) - 20:34, 10 November 2024
In chemistry, a non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed...
27 KB (3,311 words) - 07:44, 12 November 2024
chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur...
8 KB (933 words) - 06:40, 19 May 2024
triple bond in chemistry is a chemical bond between two atoms involving six bonding electrons instead of the usual two in a covalent single bond. Triple...
5 KB (516 words) - 14:46, 15 March 2024
ionic compounds. It is one of the main types of bonding, along with covalent bonding and metallic bonding. Ions are atoms (or groups of atoms) with an electrostatic...
18 KB (2,338 words) - 02:36, 8 February 2024
bond theory into computer programs, have been solved largely, and valence bond theory has seen a resurgence. According to this theory a covalent bond...
12 KB (1,519 words) - 17:16, 5 November 2024
The covalent radius, rcov, is a measure of the size of an atom that forms part of one covalent bond. It is usually measured either in picometres (pm)...
15 KB (774 words) - 09:41, 16 November 2024
structurally consist of continuous two-dimensional sheets covalently bonded within the layer, with other bond types holding the layers together. Disordered network...
4 KB (456 words) - 13:40, 24 November 2024
chemistry, a hydrogen bond (or H-bond) is primarily an electrostatic force of attraction between a hydrogen (H) atom which is covalently bonded to a more electronegative...
46 KB (5,463 words) - 19:48, 6 November 2024
the single bond. A covalent bond can also be a double bond or a triple bond. A single bond is weaker than either a double bond or a triple bond. This difference...
5 KB (600 words) - 16:47, 9 October 2024
Intermolecular force (redirect from Dipole-dipole bond)
forces – the forces which hold a molecule together. For example, the covalent bond, involving sharing electron pairs between atoms, is much stronger than...
28 KB (3,469 words) - 12:04, 9 October 2024
The covalent bond classification (CBC) method, also referred to as LXZ notation, is a way of describing covalent compounds such as organometallic complexes...
6 KB (734 words) - 09:09, 9 January 2024
pairs form a crystal structure with metallic bonding between them. Another example of a metal–metal covalent bond is the mercurous ion (Hg2+ 2). As chemistry...
24 KB (3,401 words) - 20:25, 18 January 2024
A carbon–carbon bond is a covalent bond between two carbon atoms. The most common form is the single bond: a bond composed of two electrons, one from...
9 KB (1,043 words) - 04:13, 20 September 2024
The carbon–fluorine bond is a polar covalent bond between carbon and fluorine that is a component of all organofluorine compounds. It is one of the strongest...
15 KB (1,701 words) - 19:03, 21 September 2024
Delocalized electron (category Chemical bonding)
ion or solid metal that are not associated with a single atom or a covalent bond. The term delocalization is general and can have slightly different...
4 KB (448 words) - 09:16, 12 February 2024
A carbon–oxygen bond is a polar covalent bond between atoms of carbon and oxygen.: 16–22 Carbon–oxygen bonds are found in many inorganic compounds such...
10 KB (1,026 words) - 09:33, 31 October 2024
chemistry, bond order is a formal measure of the multiplicity of a covalent bond between two atoms. As introduced by Linus Pauling, bond order is defined...
9 KB (1,260 words) - 05:21, 12 February 2023
Intramolecular force (category Chemical bonding)
ionic, covalent, and metallic — distinguished by the degree of charge separation between participating atoms. The characteristics of the bond formed can...
7 KB (795 words) - 21:19, 6 November 2024
Valence electron (category Chemical bonding)
formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with both atoms in the bond each contributing...
24 KB (2,333 words) - 14:12, 27 October 2024
In organic chemistry, a peptide bond is an amide type of covalent chemical bond linking two consecutive alpha-amino acids from C1 (carbon number one)...
12 KB (1,388 words) - 05:15, 27 April 2024
Heterolysis (chemistry) (redirect from Heterolytic bond cleavage)
cleaving/breaking a covalent bond where one previously bonded species takes both original bonding electrons from the other species. During heterolytic bond cleavage...
5 KB (641 words) - 21:53, 3 February 2024
between atoms, ions or molecules Covalent bond or molecular bond, a sharing of electron pairs between atoms Bonding molecular orbital, an attraction between...
390 bytes (82 words) - 17:35, 21 February 2019
strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals along the internuclear axis. Sigma bonding is most simply...
8 KB (918 words) - 13:02, 22 September 2024
Van Arkel–Ketelaar triangle (redirect from Bond triangle)
degrees of ionic, metallic and covalent bonding. In 1941 Van Arkel recognised three extreme materials and associated bonding types. Using 36 main group elements...
3 KB (377 words) - 13:21, 4 May 2023
Kazimierz Fajans in 1923, are used to predict whether a chemical bond will be covalent or ionic, and depend on the charge on the cation and the relative...
5 KB (534 words) - 01:02, 6 June 2024