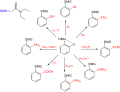
In electrophilic aromatic substitution reactions, existing substituent groups on the aromatic ring influence the overall reaction rate or have a directing...
27 KB (2,728 words) - 23:10, 24 March 2024
Electrophilic aromatic substitution (SEAr) is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced...
18 KB (2,168 words) - 20:30, 8 October 2024
protecting groups can be used to prevent electrophilic aromatic substitution. They can also be installed as directing groups to affect the position where a substitution...
5 KB (479 words) - 20:20, 30 August 2024
yellow flame, due to high C:H ratio Undergo electrophilic substitution reactions and nucleophilic aromatic substitutions Arenes are typically split into...
21 KB (2,098 words) - 00:54, 5 October 2024
Nitration (redirect from Aromatic nitration)
Regioselectivity is strongly affected by substituents on aromatic rings (see electrophilic aromatic substitution). For example, nitration of nitrobenzene...
10 KB (1,213 words) - 16:49, 2 November 2024
assistance from the meta-directing group influencing the positioning of the incoming substituent. For example, the electrophilic aromatic nitration of 1-methyl-3-nitrobenzene...
8 KB (938 words) - 01:22, 25 October 2024
Electrophilic fluorination is the combination of a carbon-centered nucleophile with an electrophilic source of fluorine to afford organofluorine compounds...
11 KB (1,345 words) - 14:57, 6 February 2024
Arene substitution pattern (redirect from Aromatic ortho position)
electrophilic aromatic substitution. Trimethylsilyl, tert-butyl, and isopropyl groups can form stable carbocations, hence are ipso directing groups....
10 KB (1,049 words) - 11:18, 3 January 2024
Electrophile (redirect from Electrophilic)
takes the form of 3 main steps shown below; Forming of a π-complex The electrophilic Br-Br molecule interacts with electron-rich alkene molecule to form...
19 KB (2,321 words) - 12:01, 2 March 2024
Directed ortho metalation (DoM) is an adaptation of electrophilic aromatic substitution in which electrophiles attach themselves exclusively to the ortho-...
9 KB (1,041 words) - 06:11, 2 November 2024
undesired side reactions with electrophilic attack, isomerization or catalytic hydration. For alkenes two protecting groups are basically known: Temporary...
57 KB (6,755 words) - 02:59, 9 November 2024
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains...
18 KB (1,913 words) - 21:16, 5 November 2024
Ester (redirect from Ester group)
monocyclic aromatic units linked by an ester group Depsipeptide, a type of ester that is a peptide in which one or more of its amide groups (−C(=O)−NH−)...
46 KB (4,840 words) - 14:46, 25 October 2024
trifluoromethyl groups to chemical compounds is actively pursued in academic research. The first to investigate trifluoromethyl groups in relationship...
43 KB (4,064 words) - 06:38, 27 April 2024
Organic chemistry (section Aromatic compounds)
to increased electrophilicity with lower pKa and increased nucleophile strength with higher pKa. More basic/nucleophilic functional groups desire to attack...
39 KB (4,396 words) - 06:25, 11 November 2024
toward electrophilic aromatic substitution. The enhanced nucleophilicity is attributed to donation pi electron density from O into the ring. Many groups can...
45 KB (4,781 words) - 17:59, 17 October 2024
Ether (redirect from Ether group)
where R and R′ represent the organyl groups. Ethers can again be classified into two varieties: if the organyl groups are the same on both sides of the oxygen...
19 KB (1,835 words) - 18:47, 14 October 2024
unsaturation for fats and other organic compounds. Aromatic compounds are subject to electrophilic halogenation: R−C6H5 + X2 → HX + R−C6H4−X This kind...
10 KB (1,112 words) - 15:24, 12 June 2024
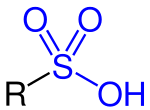
Sulfonic acid (redirect from Sulfo group)
and the arene is the nucleophile. The reaction is an example of electrophilic aromatic substitution. Alkylsulfonic acids can be prepared by many methods...
16 KB (1,935 words) - 16:58, 7 September 2024
Benzoic acid (section Aromatic ring)
Reactions of benzoic acid can occur at either the aromatic ring or at the carboxyl group. Electrophilic aromatic substitution reaction will take place mainly...
28 KB (2,451 words) - 04:19, 2 November 2024
possibilities not involving direct C–H bond cleavage by the metal include (i) generation of arylmetal species by electrophilic aromatic substitution mechanism...
36 KB (3,914 words) - 09:58, 15 September 2024
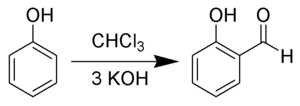
interaction favors selective ortho-formylation, consistent with other electrophilic aromatic substitution reactions. Hydroxides are not readily soluble in chloroform...
8 KB (796 words) - 09:30, 7 August 2024
Pyridine (category Aromatic bases)
distinguished for three chemical groups. With electrophiles, electrophilic substitution takes place where pyridine expresses aromatic properties. With nucleophiles...
76 KB (7,667 words) - 02:42, 23 October 2024
Indole (category Simple aromatic rings)
caused by this protonation. The most reactive position on indole for electrophilic aromatic substitution is C3, which is 1013 times more reactive than benzene...
31 KB (3,188 words) - 22:50, 16 September 2024
which is treated with a mixture of iodine and nitric acid in an electrophilic aromatic substitution. The resulting mixture of o and p-iodotoluene is then...
4 KB (305 words) - 01:39, 25 January 2024
coupling partner. Generally, coupling partners are other aromatic compounds with electron-donating groups: ArN+ 2 + Ar′H → ArN=NAr′ + H+ In practice, acetoacetic...
11 KB (1,215 words) - 15:11, 15 August 2024
is complementary to electrophilic aromatic substitution. The Sandmeyer reaction is an example of a radical-nucleophilic aromatic substitution (SRNAr)...
16 KB (1,738 words) - 18:24, 22 October 2024
Diazonium compound (category Functional groups)
arenes such as anilines and phenols, the process is an example of electrophilic aromatic substitution: [ArN2]+ + Ar'H → ArN2Ar' + H+ The deep colors of the...
32 KB (3,277 words) - 09:54, 9 September 2024
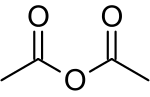
has electrophilic character, as the leaving group is carboxylate. The internal asymmetry may contribute to acetic anhydride's potent electrophilicity as...
17 KB (1,440 words) - 15:31, 14 November 2024
modification to their molecular structure. These agents typically include electrophilic groups that react readily with the net negative charge of DNA molecules...
45 KB (4,577 words) - 12:14, 27 October 2024