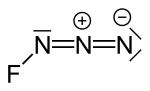Fluorine forms a great variety of chemical compounds, within which it always adopts an oxidation state of −1. With other atoms, fluorine forms either polar...
85 KB (8,550 words) - 23:52, 26 August 2024
War II. Owing to the expense of refining pure fluorine, most commercial applications use fluorine compounds, with about half of mined fluorite used in steelmaking...
157 KB (15,334 words) - 14:36, 6 November 2024
Organofluorine chemistry (redirect from Hydro fluoro compounds)
the chemistry of organofluorine compounds, organic compounds that contain a carbon–fluorine bond. Organofluorine compounds find diverse applications ranging...
41 KB (4,534 words) - 14:34, 22 September 2024
Fluorocarbon (section Electrochemical fluorination)
Fluorocarbons are chemical compounds with carbon-fluorine bonds. Compounds that contain many C-F bonds often have distinctive properties, e.g., enhanced[clarification...
25 KB (2,335 words) - 14:15, 28 October 2024
Hydrogen fluoride (redirect from Fluorine hydride)
industrial source of fluorine, often in the form of hydrofluoric acid, and is an important feedstock in the preparation of many important compounds including pharmaceuticals...
15 KB (1,376 words) - 20:39, 18 October 2024
Halogenation (redirect from Fluorination)
hazards of handling fluorine gas. Many commercially important organic compounds are fluorinated using this technology. Unsaturated compounds, especially alkenes...
10 KB (1,112 words) - 15:24, 12 June 2024
Halogen (redirect from Fluorine family)
They include PCBs, PBDEs, and perfluorinated compounds (PFCs), as well as numerous other compounds. Fluorine reacts vigorously with water to produce oxygen...
52 KB (5,499 words) - 17:27, 19 November 2024
Fluoride (/ˈflʊəraɪd, ˈflɔːr-/) is an inorganic, monatomic anion of fluorine, with the chemical formula F− (also written [F]− ), whose salts are typically...
47 KB (5,060 words) - 17:55, 21 November 2024
everyday life, fluorine-containing compounds such as fluorite occur naturally as minerals. Naturally occurring organofluorine compounds are extremely rare...
55 KB (5,787 words) - 10:05, 5 January 2024
Fluorine-19 nuclear magnetic resonance spectroscopy (fluorine NMR or 19F NMR) is an analytical technique used to detect and identify fluorine-containing...
15 KB (1,220 words) - 01:36, 5 October 2024
number of xenon compounds have been discovered and described. Almost all known xenon compounds contain the electronegative atoms fluorine or oxygen. The...
22 KB (2,491 words) - 15:37, 1 November 2024
Electrophilic fluorination is the combination of a carbon-centered nucleophile with an electrophilic source of fluorine to afford organofluorine compounds. Although...
11 KB (1,345 words) - 14:57, 6 February 2024
inorganic compound of fluorine and oxygen with the chemical formula O6F2. The compound is one of many known oxygen fluorides. The compound can be prepared...
3 KB (216 words) - 08:43, 11 September 2023
Bromine (section Chemistry and compounds)
electronegativity higher than bromine's (oxygen, nitrogen, fluorine, and chlorine), so that the resultant binary compounds are formally not bromides but rather oxides...
67 KB (7,719 words) - 23:39, 21 November 2024
greenhouse effect thousands of times stronger than that of CO2. Fluorine-based compounds such as sulphur hexafluoride and perfluorocarbons are preferable...
63 KB (6,290 words) - 22:58, 20 November 2024
a mistaken identification. Krypton compounds with other than Kr–F bonds (compounds with atoms other than fluorine) have also been described. KrF2 reacts...
31 KB (3,435 words) - 15:37, 1 November 2024
that fluorine was a bound element within compounds, similar to chlorine. Fluorite was determined to be calcium fluoride. Because of fluorine's tight...
22 KB (2,338 words) - 23:16, 15 August 2024
The carbon–fluorine bond is a polar covalent bond between carbon and fluorine that is a component of all organofluorine compounds. It is one of the strongest...
15 KB (1,701 words) - 19:03, 21 September 2024
Chlorine fluoride (redirect from Fluorine chloride)
fluoride is an interhalogen compound containing only chlorine and fluorine. National Pollutant Inventory - Fluoride compounds fact sheet NIST Standard Reference...
2 KB (24 words) - 13:25, 5 August 2017
Fluorine azide or triazadienyl fluoride is a yellow green gas composed of nitrogen and fluorine with formula FN3. Its properties resemble those of ClN3...
8 KB (728 words) - 22:43, 21 July 2024
fluorocarbon-based organofluorine compounds. The general approach represents an application of electrosynthesis. The fluorinated chemical compounds produced by ECF are...
5 KB (669 words) - 09:20, 12 July 2024
Dioxygen difluoride (redirect from Fluorine dioxide)
Dioxygen difluoride is a compound of fluorine and oxygen with the molecular formula O2F2. It can exist as an orange-red colored solid which melts into...
11 KB (1,064 words) - 16:20, 31 October 2024
Calcium fluoride (category Calcium compounds)
Calcium fluoride is the inorganic compound of the elements calcium and fluorine with the formula CaF2. It is a white solid that is practically insoluble...
10 KB (843 words) - 01:08, 8 November 2024
Hydrofluoric acid (category Chemical articles with multiple compound IDs)
boiling point near room temperature. It is used to make most fluorine-containing compounds; examples include the commonly used pharmaceutical antidepressant...
21 KB (2,096 words) - 08:43, 2 November 2024
Interhalogen (redirect from Interhalogen compounds)
In chemistry, an interhalogen compound is a molecule which contains two or more different halogen atoms (fluorine, chlorine, bromine, iodine, or astatine)...
20 KB (2,322 words) - 16:38, 1 November 2024
process, gaseous inorganic chlorine compounds are either not emitted at all, or in very small quantities only. Of the fluorine present in rotary kilns, 90 to...
48 KB (7,205 words) - 16:14, 30 May 2024
they strengthen as more carbon–fluorine bonds are added to the same carbon. In the one-carbon organofluorine compounds represented by molecules of fluoromethane...
14 KB (1,151 words) - 21:11, 11 July 2024
Nitrogen trifluoride (category Nitrogen(III) compounds)
authors list (link) Klapötke, Thomas M. (2006). "Nitrogen–fluorine compounds". Journal of Fluorine Chemistry. 127 (6): 679–687. doi:10.1016/j.jfluchem.2006...
22 KB (1,964 words) - 06:53, 26 September 2024
Xenon tetrafluoride (category Xenon(IV) compounds)
; Stein, L.; Studier, M. H.; Weeks, J. L.; Zirin, M. H. (1962). "Fluorine Compounds of Xenon and Radon". Science. 138 (3537): 136–138. Bibcode:1962Sci...
11 KB (920 words) - 03:58, 17 June 2024
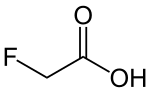
Fluoroacetic acid (category Fluorine-containing natural products)
Fluoroacetic acid. Timperley, Christopher M. (2000). "Highly-toxic fluorine compounds". Fluorine Chemistry at the Millennium. pp. 499–538. doi:10.1016/B978-008043405-6/50040-2...
6 KB (417 words) - 23:57, 1 September 2024