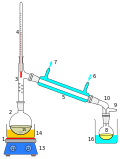
Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid)...
26 KB (3,008 words) - 03:36, 18 November 2024
The vapor pressure of water is the pressure exerted by molecules of water vapor in gaseous form (whether pure or in a mixture with other gases such as...
13 KB (971 words) - 08:36, 13 December 2024
means that the vapor can be condensed to a liquid by increasing the pressure on it without reducing the temperature of the vapor. A vapor is different from...
7 KB (753 words) - 15:21, 9 June 2024
Reid vapor pressure (RVP) is a common measure of the volatility of gasoline and other petroleum products. It is defined as the absolute vapor pressure exerted...
4 KB (625 words) - 22:39, 1 April 2024
Boiling point (redirect from Atmospheric pressure boiling point)
temperature at which the vapor pressure of a liquid equals the pressure surrounding the liquid and the liquid changes into a vapor. The boiling point of...
19 KB (2,335 words) - 12:30, 26 November 2024
metal cap. They include the sodium-vapor lamp that is the gas-discharge lamp in street lighting. The low-pressure sodium arc discharge lamp was first...
28 KB (3,200 words) - 09:11, 7 December 2024
Raoult's law (redirect from Vapor pressure lowering)
1887, it states that the partial pressure of each component of an ideal mixture of liquids is equal to the vapor pressure of the pure component (liquid or...
16 KB (2,376 words) - 20:03, 19 December 2024
of a vapor in contact with its liquid, especially at equilibrium, is often expressed in terms of vapor pressure, which will be a partial pressure (a part...
22 KB (3,245 words) - 19:11, 29 September 2024
amount of substance in the gas mixture Vapor pressure is the pressure of a vapor in equilibrium with its non-vapor phases (i.e., liquid or solid). Most...
21 KB (2,402 words) - 11:32, 11 October 2024
equilibrium vapor pressure; 100% relative humidity occurs when the partial pressure of water vapor is equal to the equilibrium vapor pressure. This condition...
59 KB (6,454 words) - 19:37, 10 December 2024
Humidity (section Pressure dependence)
Humidity depends on the temperature and pressure of the system of interest. The same amount of water vapor results in higher relative humidity in cool...
69 KB (7,393 words) - 06:01, 25 December 2024
critical point. Vapor Pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its solid or liquid state. The vapor pressure of a solvent...
13 KB (2,107 words) - 06:06, 24 August 2024
using high voltage pulses as with high-pressure sodium vapor lamps. Self-ballasted (SB) lamps are mercury-vapor lamps with a tungsten filament inside connected...
23 KB (2,772 words) - 22:50, 17 December 2024
Volatility (chemistry) (section Vapor pressure)
value, but it is often described using vapor pressures or boiling points (for liquids). High vapor pressures indicate a high volatility, while high boiling...
10 KB (1,118 words) - 03:59, 14 August 2024
When a molecule near the surface absorbs enough energy to overcome the vapor pressure, it will escape and enter the surrounding air as a gas. When evaporation...
15 KB (1,800 words) - 16:53, 29 July 2024
applications. Crude oil stabilisation, a partial distillation to reduce the vapor pressure of crude oil, which thus is safe to store and to transport, and thereby...
71 KB (8,813 words) - 11:30, 1 January 2025
Azeotrope (redirect from Pressure swing distillation)
compositions in liquid phase and vapor phases, in vapour-liquid equilibrium and Dalton's law the equality of pressures for total pressure being equal to the sum...
34 KB (4,407 words) - 23:15, 6 December 2024
T},} where v p sat {\displaystyle vp_{\text{sat}}} is the saturation vapor pressure in PSI, A = − 1.0440397 × 10 4 {\displaystyle A=-1.0440397\times 10^{4}}...
6 KB (790 words) - 17:11, 23 September 2024
CRC Press. Boca Raton, Florida, 2003; Section 6, Fluid Properties; Vapor Pressure Uncertainties of several degrees should generally be assumed. (e) Indicates...
118 KB (1,365 words) - 16:47, 12 November 2023
True vapor pressure (TVP) is a common measure of the volatility of petroleum distillate fuels. It is defined as the equilibrium partial pressure exerted...
1 KB (157 words) - 18:12, 10 September 2021
W. F.; Egan, C. J. (1937). "Carbon Dioxide. The Heat Capacity and Vapor Pressure of the Solid. The Heat of Sublimation. Thermodynamic and Spectroscopic...
25 KB (817 words) - 20:13, 28 December 2024
the liquid evaporates. Vapor pressure is a major determinant of the flash point and flame point, with higher vapor pressures leading to lower flash points...
34 KB (3,640 words) - 00:02, 12 December 2024
because of surface tension, the vapor pressure for small droplets of liquid in suspension is greater than standard vapor pressure of that same liquid when the...
72 KB (8,812 words) - 07:53, 29 November 2024
In polymer chemistry, vapor phase osmometry (VPO), also known as vapor-pressure osmometry, is an experimental technique for the determination of a polymer's...
5 KB (616 words) - 21:23, 24 September 2024
Chemical vapor deposition (CVD) is a vacuum deposition method used to produce high-quality, and high-performance, solid materials. The process is often...
42 KB (5,006 words) - 15:45, 8 December 2024
Heat pipe (redirect from Vapor Chamber Technology)
thermally conductive solid surface turns into a vapor by absorbing heat from that surface. The vapor then travels along the heat pipe to the cold interface...
59 KB (7,644 words) - 05:19, 18 December 2024
of vapor pressure, a liquid boils when its vapor pressure equals the surrounding pressure. A nonvolatile solute lowers the solvent’s vapor pressure, meaning...
9 KB (1,037 words) - 19:38, 3 December 2024
and pressure. Reliability of data general note. Lange's Handbook of Chemistry, 10th ed. pp 1669–1674 Parks, J. S.; B. Barton (1928). "Vapor pressure data...
10 KB (170 words) - 07:13, 21 July 2023
Henry's law (redirect from Vapor-liquid distribution ratio)
the constant of proportionality is the vapor pressure of the pure substance (Raoult's law). The vapor pressure of the solute is also proportional to the...
31 KB (4,888 words) - 06:38, 30 December 2024
mixture of ideal gases. In this case, the partial pressure of water vapor is known as the vapor pressure. Using this method, error in the density calculation...
17 KB (2,791 words) - 21:28, 21 November 2024