4.19 million vaccines were then being administered daily, only 22.3 percent of people in low-income countries had received at least a first vaccine by...
156 KB (13,918 words) - 21:34, 29 September 2024
most COVID‑19 vaccines were two-dose vaccines, with the exception single-dose vaccines Convidecia and the Janssen COVID‑19 vaccine, and vaccines with three-dose...
207 KB (23,491 words) - 04:04, 30 December 2024
The Pfizer–BioNTech COVID-19 vaccine, sold under the brand name Comirnaty, is an mRNA-based COVID-19 vaccine developed by the German biotechnology company...
260 KB (22,123 words) - 05:02, 30 December 2024
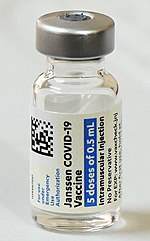
The Janssen COVID‑19 vaccine, (Ad26.COV2.S) sold under the brand name Jcovden, is a COVID‑19 vaccine that was developed by Janssen Vaccines in Leiden,...
141 KB (11,331 words) - 06:12, 24 September 2024
In many countries a variety of unfounded conspiracy theories and other misinformation about COVID-19 vaccines have spread based on misunderstood or misrepresented...
132 KB (12,911 words) - 03:13, 18 November 2024
Sanofi–GSK COVID-19 vaccine, sold under the brand name VidPrevtyn Beta, is a COVID-19 vaccine developed by Sanofi Pasteur and GSK. The Sanofi–GSK COVID‑19 vaccine...
19 KB (1,436 words) - 03:58, 29 November 2024
authorizations (CMA) for the mRNA vaccines BNT162b2 and mRNA1273 (Moderna Covid‑19 vaccine). The assessments of the vaccines are scheduled to proceed under...
122 KB (11,017 words) - 03:34, 5 January 2025
from a disease. COVID-19 vaccine card COVID-19 vaccine Deployment of COVID-19 vaccines Electronic health record Living with COVID-19 Patient record access...
92 KB (8,794 words) - 15:22, 22 December 2024
COVID-19 vaccine Deployment of COVID-19 vaccines The table this note applies to is updated daily by a bot. For more info see Template:COVID-19 data/Cite...
13 KB (1,536 words) - 14:49, 21 December 2024
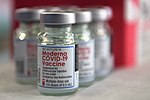
The Moderna COVID‑19 vaccine, sold under the brand name Spikevax, is a COVID-19 vaccine developed by the American company Moderna, the United States National...
177 KB (13,698 words) - 16:52, 23 October 2024
13% of those eligible ha[d] received an updated booster dose." Deployment of COVID-19 vaccines COVID-19 pandemic in the United States COVID-19 vaccine Ohio...
134 KB (10,725 words) - 02:06, 15 December 2024
Stemirna COVID-19 vaccine is a COVID-19 vaccine candidate developed by Stemirna Therapeutics. "Patent Landscape Report COVID-19-related vaccines and therapeutics"...
3 KB (113 words) - 09:01, 24 September 2024
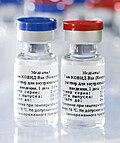
Gam-COVID-Vac (Russian: Гам-КОВИД-Вак, the name under which it is legally registered and produced) is an adenovirus viral vector vaccine for COVID-19 developed...
154 KB (13,917 words) - 20:05, 2 January 2025
COVID-19 pages. COVID-19 portal List of epidemics List of deaths due to COVID-19 – notable individual deaths COVID-19 vaccine Deployment of COVID-19 vaccines...
18 KB (1,603 words) - 03:22, 28 December 2024
Vaxart COVID-19 vaccine is a COVID-19 vaccine candidate developed by Vaxart. "Patent Landscape Report COVID-19-related vaccines and therapeutics" (PDF)...
2 KB (108 words) - 10:50, 24 September 2024
COVID-19 vaccines has existed since the early stages of the vaccines' development. COVID-19 vaccine-hesitant people are not necessarily anti-vaccine....
40 KB (3,771 words) - 01:07, 31 August 2024
Oxford–AstraZeneca COVID‑19 vaccine, sold under the brand names Covishield and Vaxzevria among others, is a viral vector vaccine for the prevention of COVID-19. It was...
214 KB (17,080 words) - 15:51, 27 September 2024
Inovio COVID-19 vaccine is a COVID-19 vaccine candidate developed by Inovio Pharmaceuticals. In February 2020 after receiving details of the genetic sequence...
9 KB (880 words) - 06:15, 24 September 2024
The Novavax COVID-19 vaccine, sold under the brand names Nuvaxovid and Covovax, among others, is a subunit COVID-19 vaccine developed by Novavax and the...
75 KB (5,760 words) - 02:39, 14 December 2024
variety of technologies are being used to formulate vaccines against COVID-19. The development and deployment of mRNA vaccines and viral vector vaccines has...
98 KB (19,258 words) - 04:46, 25 November 2024
new COVID-19 vaccines". "US FDA Signals New COVID-19 Vaccines Could Be Authorized With Just Immunogenicity Studies". 7 September 2021. "FDA Review of Effectiveness...
15 KB (1,455 words) - 06:02, 24 September 2024
introduced in Europe affected more than 250 million people. Despite deployment of COVID-19 vaccines, Europe became the pandemic's epicentre once again in late...
63 KB (5,722 words) - 22:34, 11 December 2024
COVID-19 vaccine, also known as BBIBP-CorV, the Sinopharm COVID-19 vaccine, or BIBP vaccine, is one of two whole inactivated virus COVID-19 vaccines developed...
90 KB (8,588 words) - 08:11, 24 September 2024
of two of the Pfizer–BioNTech COVID-19 vaccine. There are three vaccines currently in use; following approval of the Pfizer–BioNTech COVID-19 vaccine...
118 KB (9,094 words) - 19:26, 12 September 2024
distribute vaccines to individual provincial and territorial governments who in turn administer authorized COVID-19 vaccines during the COVID-19 pandemic...
185 KB (14,787 words) - 03:28, 5 January 2025
forecast was 120 million. COVID-19 pandemic in France Deployment of COVID-19 vaccines EU Digital COVID Certificate Watson, Oliver J; Barnsley, Gregory; Toor...
47 KB (687 words) - 13:22, 24 June 2024
Valneva COVID-19 vaccine is a COVID-19 vaccine developed by French biotechnology company Valneva SE in collaboration with the American biopharmaceutical...
19 KB (1,339 words) - 05:11, 21 November 2024
introduced in Europe affected more than 250 million people. Despite deployment of COVID-19 vaccines, Europe became the pandemic's epicentre once again in late...
204 KB (22,846 words) - 20:24, 24 September 2024
vaccines doses have arrived to the country. COVID-19 pandemic in Bosnia and Herzegovina Deployment of COVID-19 vaccines B.R. (12 February 2021). "Počela vakcinacija...
4 KB (189 words) - 20:15, 16 May 2024
MD (31 December 2023). "Can protein vaccines for COVID-19 win over the vaccine-hesitant?". Expert Review of Vaccines. 22 (1): 210–212. doi:10.1080/14760584...
368 KB (32,718 words) - 17:52, 31 December 2024