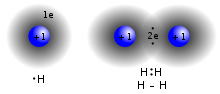
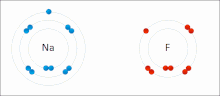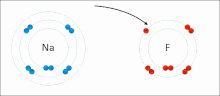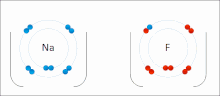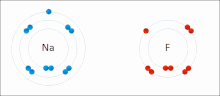
The higher the associated electronegativity, the more an atom or a substituent group attracts electrons. Electronegativity serves as a simple way to quantitatively...
35 KB (4,360 words) - 15:05, 21 October 2024
e Periodic table of electronegativity by Pauling scale → Atomic radius decreases → Ionization energy increases → Electronegativity increases → See also:...
13 KB (494 words) - 15:52, 1 November 2024
Periodic trends (section Electronegativity)
known as electronegativity. It is a dimensionless quantity because it is only a tendency. The most commonly used scale to measure electronegativity was designed...
15 KB (1,602 words) - 03:15, 25 October 2024
difference in electronegativity between the two atoms is less than 0.5 Polar bonds generally occur when the difference in electronegativity between the...
24 KB (2,751 words) - 20:34, 10 November 2024
organized by other properties, such as atomic weight, density, and electronegativity. For more detailed information about the origins of element names...
2 KB (439 words) - 14:28, 13 October 2024
developed the idea of electronegativity equalization, stating two bonding atoms will equalize their Mulliken electronegativity. He would later further...
4 KB (386 words) - 07:31, 10 November 2024
strong bond differ due to the difference in electronegativity of the constituent elements. Electronegativity is the tendency for an atom of a given chemical...
40 KB (4,872 words) - 13:33, 22 September 2024
triangle, Norman's quantitative triangle) used electronegativity of compounds. Nowadays, electronegativity triangles are mostly used to rate the chemical...
3 KB (377 words) - 13:21, 4 May 2023
effect. Covalent bonds can be polarized depending on the relative electronegativity of the two atoms forming the bond. The electron cloud in a σ-bond...
10 KB (1,258 words) - 17:02, 2 October 2024
affected by the electronegativity of the connected atoms which determines the chemical polarity of the bond. Two atoms with equal electronegativity will make...
28 KB (3,673 words) - 14:01, 24 October 2024
metals and nonmetals depends on the electronegativity difference. Ionicity is possible when the electronegativity difference is high enough (e.g. Li3N...
19 KB (2,487 words) - 01:45, 3 November 2024
Periodic table (section Electronegativity)
scale cannot measure its electronegativity because it does not form covalent bonds with most elements. An element's electronegativity varies with the identity...
250 KB (26,986 words) - 04:50, 11 November 2024
substituent electronegativities and it is for this work that Bent's rule takes its name. Bent's original paper considers the group electronegativity of the...
38 KB (4,250 words) - 13:50, 4 October 2024
chemical bonds are shared equally between atoms, regardless of relative electronegativity. In simple terms, formal charge is the difference between the number...
9 KB (1,050 words) - 02:14, 19 September 2024
character – that is, a bond in which there is a large difference in electronegativity between the two atoms, causing the bonding to be more polar (ionic)...
18 KB (2,338 words) - 02:36, 8 February 2024
electron affinities was used by Robert S. Mulliken to develop an electronegativity scale for atoms, equal to the average of the electrons affinity and...
22 KB (1,468 words) - 21:34, 21 October 2024
Radical (chemistry) (section Electronegativity)
higher electronegativity of the carbon atom (due to the close proximity of s orbitals to the nucleus), and the greater the electronegativity the less...
40 KB (4,613 words) - 06:59, 17 October 2024
polydimethylsiloxane. On the Pauling electronegativity scale, silicon has an electronegativity of 1.90 and oxygen 3.44. The electronegativity difference between the...
11 KB (1,124 words) - 12:41, 22 September 2024
extract with the electronegativity values of metals. Wulfsberg distinguishes: very electropositive metals with electronegativity values below 1.4 ...
19 KB (1,028 words) - 04:02, 16 November 2024
higher electronegativity; in a bond between two atoms of the same element, the electrons are divided equally. This is because most electronegativity scales...
47 KB (8,698 words) - 02:26, 27 October 2024
densities and high electronegativity. The accompanying table, using a threshold of 7 g/cm3 for density and 1.9 for electronegativity (revised Pauling)...
186 KB (16,953 words) - 11:05, 23 October 2024
the negative of the electronegativity (χ) definition on the Mulliken scale: μ = −χ. The hardness and Mulliken electronegativity are related as 2 η =...
19 KB (2,118 words) - 13:31, 25 October 2024
technical standards Electroless nickel plating, a chemical technique Electronegativity, chemical tendency to attract electrons Engrailed (gene), a gene involved...
2 KB (333 words) - 22:30, 16 November 2024
They can be seen at the bottom right in the accompanying plot of electronegativity values and melting points. The boundaries of the category are not...
124 KB (15,306 words) - 16:20, 28 October 2024
letter Chi because of its Χ-shape. In chemistry, the mole fraction and electronegativity may be denoted by the lowercase χ {\displaystyle \chi } . In physics...
7 KB (838 words) - 03:08, 10 November 2024
strongly electronegative elements, and therefore nitric acid, sulfuric acid, and perchloric acid, are strong acids. If, however, the electronegativity of X...
22 KB (1,761 words) - 14:55, 11 October 2024
most useful for covalently bonded compounds. In ionic compounds, the electronegativity of the two atoms bonding together has a major effect on their bond...
9 KB (1,318 words) - 02:44, 29 April 2024
sulfide is a network solid made up of silver (electronegativity of 1.98) and sulfur (electronegativity of 2.58) where the bonds have low ionic character...
11 KB (970 words) - 15:20, 5 November 2024
tetrafluoride) are some of the most unreactive organic compounds. The high electronegativity of fluorine (4.0 for fluorine vs. 2.5 for carbon) gives the carbon–fluorine...
15 KB (1,701 words) - 19:03, 21 September 2024
Photoredox catalysis (section Ligand electronegativity)
effect. According to the Pauling scale of electronegativity, both ruthenium and iridium have an electronegativity of 2.2. If this was the sole factor relevant...
50 KB (6,209 words) - 14:32, 1 November 2024