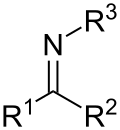
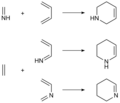
In organic chemistry, an imine (/ɪˈmiːn/ or /ˈɪmɪn/) is a functional group or organic compound containing a carbon–nitrogen double bond (C=N). The nitrogen...
23 KB (2,633 words) - 07:34, 30 May 2024
Sydnone imine is a mesoionic heterocyclic aromatic chemical compound. Sydnone imine is the imine of sydnone where the keto functional group of sydnone...
942 bytes (48 words) - 16:24, 20 April 2024
Methylene imine is an organic compound with the chemical formula H2C=NH. The simplest imine, it is a stable, colorless gas that has been detected throughout...
3 KB (176 words) - 21:34, 24 March 2024
An imine reductase (IRED) is an enzyme that reduces imines to amines. This family of enzymes is employed in the industrial production of amine-containing...
7 KB (758 words) - 08:04, 10 August 2024
Benzophenone imine is an organic compound with the formula of (C6H5)2C=NH. A pale yellow liquid, benzophenone imine is used as a reagent in organic synthesis...
5 KB (399 words) - 04:32, 15 June 2024
Aza-Diels–Alder reaction (redirect from Imine_Diels–Alder_reaction)
process places the imine lone pair (or coordinated Lewis acid) in an exo position. Thus, (E) imines, in which the lone pair and larger imine carbon substituent...
10 KB (972 words) - 11:37, 13 August 2024
imines, being either secondary ketimines or secondary aldimines depending on their structure. Anil refers to a common subset of Schiff bases: imines derived...
9 KB (880 words) - 13:55, 24 May 2024
Amine (section Conversion to imines)
the production of dyes. Imine formation is an important reaction. Primary amines react with ketones and aldehydes to form imines. In the case of formaldehyde...
35 KB (3,695 words) - 10:22, 30 July 2024
Acetone imine, or 2-propanimine is an organic compound and an imine with the chemical formula (CH3)2CNH. It is a volatile and flammable liquid at room...
4 KB (223 words) - 11:24, 18 January 2024
involves the conversion of a carbonyl group to an amine via an intermediate imine. The carbonyl group is most commonly a ketone or an aldehyde. It is a common...
21 KB (2,134 words) - 00:28, 26 May 2024
Azetidine (redirect from Trimethylene imine)
Azetidine Systematic IUPAC name Azacyclobutane Other names Azetane Trimethylene imine 1,3-Propylenimine Identifiers CAS Number 503-29-7 Y 3D model (JSmol) Interactive...
5 KB (311 words) - 11:45, 6 August 2022
Polyethylenimine (redirect from Poly(ethylene imine))
Polyethylenimine (PEI) or polyaziridine is a polymer with repeating units composed of the amine group and two carbon aliphatic CH2CH2 spacers. Linear polyethyleneimines...
22 KB (2,400 words) - 20:53, 11 June 2024
An imine N-oxide may refer to: An oxime (>C=NOH) A nitrone (>C=N(O)-) This disambiguation page lists articles associated with the title Imine N-oxide....
96 bytes (49 words) - 20:50, 30 April 2024
Propyleneimine (redirect from Propylene imine)
Propyleneimine (or propylene imine) is the organic compound with the formula CH3CH(NH)CH2. It is a secondary amine and the smallest chiral aziridine (ring...
4 KB (306 words) - 15:08, 12 July 2024
Aziridine (redirect from Ethylene imine)
Aziridine is an organic compound consisting of the three-membered heterocycle C2H5N. It is a colorless, toxic, volatile liquid that is of significant practical...
8 KB (564 words) - 13:57, 17 June 2024
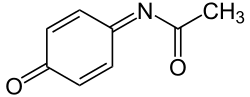
NAPQI (redirect from N-acetyl-p-benzo-quinone imine)
NAPQI, also known as NAPBQI or N-acetyl-p-benzoquinone imine, is a toxic byproduct produced during the xenobiotic metabolism of the analgesic paracetamol...
7 KB (725 words) - 22:14, 13 February 2024
any primary or secondary amine to produce resonance stabilized imine (iminium ion or imine salt). The addition of a carbanion from a CH acidic compound...
2 KB (223 words) - 20:34, 6 August 2020
determines the rate of reaction. More electron rich imines reduce at faster rates than electron poor imines. The resulting iminium center undergoes nucleophilic...
23 KB (2,669 words) - 14:59, 5 June 2024
Covalent organic framework (section Imine condensation)
high temperature (400 °C)). CTF-1 is a good example of this chemistry. The imine condensation reaction which eliminates water (exemplified by reacting aniline...
49 KB (5,592 words) - 10:27, 15 August 2024
of carbon dioxide. An ammonium ion is added forming an imine and releasing ammonia. The imine goes through hydrolysis to form the amine, which is depicted...
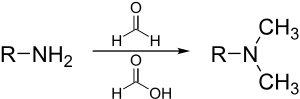
6 KB (635 words) - 05:39, 24 May 2024
of the amine begins with imine formation with formaldehyde. The formic acid acts as a source of hydride and reduces the imine to a secondary amine. Loss...
4 KB (371 words) - 23:50, 14 February 2024
N-Sulfinyl imines (N-sulfinylimines, sulfinimines, thiooxime S-oxides) are a class of imines bearing a sulfinyl group attached to nitrogen. These imines display...
15 KB (1,583 words) - 03:48, 22 October 2022
state imine. This strategy was developed by the laboratory Sivaguru and co-workers to overcome the shortcomings involving direct excitation of imines. Traditionally...
1 KB (115 words) - 03:17, 23 July 2023
In organic chemistry, an oxime is an organic compound belonging to the imines, with the general formula RR’C=N−OH, where R is an organic side-chain and...
15 KB (1,654 words) - 00:59, 6 July 2024
through an intermediate imine. The carbonyl is first treated with ammonia to promote imine formation by nucleophilic attack. The imine is then reduced to an...
9 KB (792 words) - 14:48, 1 April 2024
derived structures such as the nucleobases guanine, thymine, and cytosine imine – imine, e.g., during pyridoxal phosphate catalyzed enzymatic reactions R1R2C(=NCHR3R4)...
14 KB (1,483 words) - 08:12, 7 February 2024
In organic chemistry, an imino acid is any molecule that contains both imine (>C=NH) and carboxyl (-C(=O)-OH) functional groups. Imino acids are structurally...
4 KB (383 words) - 01:51, 30 June 2024
the unsubstituted porphine; D, a mixed amine/imine, the Curtis macrocycle; E, the related enamine/imine Jäger macrocycle, and F, the tetracarboxylate-derivative...
28 KB (3,320 words) - 13:08, 12 March 2024
side, and the imine and water on the other. However, due to aromatic conjugation between the imine group and benzene ring, the imines are relatively...
6 KB (563 words) - 12:18, 29 April 2024
Polyimine (category Imines)
Polyimines are classified as polymer materials that contain imine groups, which are characterised by a double bond between a carbon and nitrogen atom...
8 KB (888 words) - 00:51, 26 June 2024