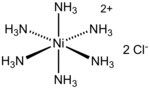
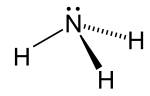
In coordination chemistry, metal ammine complexes are metal complexes containing at least one ammonia (NH3) ligand. "Ammine" is spelled this way for historical...
16 KB (1,787 words) - 12:15, 19 July 2024
Some of the simplest members of such complexes are described in metal aquo complexes, metal ammine complexes, Examples: [Co(EDTA)]−, [Co(NH3)6]3+, [Fe(C2O4)3]3-...
57 KB (5,548 words) - 12:34, 17 November 2024
[LnMNO] + OH− The reaction is reversible in some cases. In some metal-ammine complexes, the ammonia ligand can be oxidized to nitrosyl: H2O + [Ru(terpy)(bipy)(NH3)]+...
17 KB (2,019 words) - 15:22, 2 September 2024
2 H2O Phosphines are L-type ligands. Unlike most metal ammine complexes, metal phosphine complexes tend to be lipophilic, displaying good solubility in...
14 KB (1,418 words) - 17:55, 22 June 2024
complex. For example, water exchange in [Al(H2O)5OH]2+ is 20000 times faster than in [Al(H2O)6]3+. Hydration number Ligand field theory Metal ammine complex...
15 KB (1,551 words) - 19:45, 27 October 2024
Hexaamminecobalt(III) chloride (category Ammine complexes)
of coordination chemistry, Alfred Werner. The cation itself is a metal ammine complex with six ammonia ligands attached to the cobalt(III) ion. [Co(NH3)6]3+...
8 KB (665 words) - 21:00, 6 August 2024
Hexaamminenickel chloride (category Ammine complexes)
[Ni(NH3)6]Cl2. It is the chloride salt of the metal ammine complex [Ni(NH3)6]2+. The cation features six ammonia (called ammines in coordination chemistry) ligands...
4 KB (256 words) - 20:17, 6 August 2024
In organometallic chemistry, transition metal complexes of nitrite describes families of coordination complexes containing one or more nitrite (−NO2) ligands...
9 KB (1,050 words) - 15:50, 30 May 2024
(disambiguation) Amino (disambiguation) Anime, Japanese animation Metal ammine complex This disambiguation page lists articles associated with the title...
419 bytes (87 words) - 16:31, 31 October 2022
cationic metal ammine complexes such as [Pt(NH3)6]4+ spontaneously convert to the amido derivative: [Pt(NH3)6]4+ ↔ [Pt(NH3)5(NH2)]3+ + H+ Transition metal amides...
7 KB (773 words) - 10:45, 29 June 2024
general method for the determination of stability constants of metal-ammine complexes in 1941. The reasons why this occurred at such a late date, nearly...
60 KB (7,604 words) - 15:10, 15 November 2024
prominent examples of metal-ammine-chlorides. As indicated in the table below, many hydrates of metal chlorides are molecular complexes. These compounds are...
61 KB (3,477 words) - 14:14, 21 May 2024
Hexaammineplatinum(IV) chloride (category Ammine complexes)
[Pt(NH3)6]Cl4. It is the chloride salt of the metal ammine complex [Pt(NH3)6]4+. The cation features six ammonia (called ammines in coordination chemistry) ligands...
3 KB (182 words) - 21:00, 6 August 2024
Schweizer's reagent (category Ammine complexes)
Schweizer's reagent is a metal ammine complex with the formula [Cu(NH3)4(H2O)2](OH)2. This deep-blue compound is used in purifying cellulose. This salt...
7 KB (734 words) - 16:45, 20 November 2024
which features a pair of S-bonded thiosulfate ligands. Simple aquo and ammine complexes are also known. Three binding modes are common: monodentate (κ1-),...
6 KB (714 words) - 20:45, 4 July 2024
A transition metal imidazole complex is a coordination complex that has one or more imidazole ligands. Complexes of imidazole itself are of little practical...
9 KB (1,029 words) - 04:21, 16 September 2024
Azanide (section Alkali metal derivatives)
Transition metal complexes of the amido ligand are often produced by salt metathesis reaction or by deprotonation of metal ammine complexes. Bergstrom...
4 KB (271 words) - 11:05, 22 October 2024
Rhodium(III) chloride (section Coordination complexes)
chloride, [RhCl(NH3)5]Cl2. As for other metal-ammine complexes, the term "ammine" refers to ammonia bound to a metal ion as a ligand. Zinc reduction of this...
19 KB (1,917 words) - 17:56, 26 July 2024
dissolves in excess aqueous ammonia to form a colorless, water-soluble ammine complex. One major use is as an absorbent in surgical dressings. It is also...
4 KB (348 words) - 10:30, 27 August 2024
Pentaamine(dinitrogen)ruthenium(II) chloride (category Ammine complexes)
the pi backbonding, the donation of metal d-electrons into the N2 π* orbitals. The related metal ammine complex [Os(NH3)5(N2)]2+ is also known. The dinitrogen...
5 KB (466 words) - 16:05, 7 April 2024
forming metal ammine complexes. For historical reasons, ammonia is named ammine in the nomenclature of coordination compounds. One notable ammine complex is...
139 KB (14,988 words) - 17:19, 20 November 2024
Magnus's green salt (category Ammine complexes)
"Vauquelin’s salt". Magnus's green salt was one of the first examples of a metal ammine complex. Atoji, Masao; Richardson, James W.; Rundle, R. E. (June 1957). "Pt(NH3)4PtCl41"...
5 KB (455 words) - 16:09, 30 November 2023
coordination chemistry and organometallic chemistry, transition metal imido complexes is a coordination compound containing an imido ligand. Imido ligands...
6 KB (514 words) - 16:47, 30 September 2023
like most ions, have integer oxidation states. For example, ruthenium ammine complexes are typically +2 or +3. The fact that the oxidation states are half-integer...
4 KB (464 words) - 15:15, 18 December 2023
Ligand (section Metal–ligand multiple bond)
in the cobalt ammine chlorides and to explain many of the previously inexplicable isomers. He resolved the first coordination complex called hexol into...
35 KB (3,307 words) - 14:02, 22 November 2024
Zinc chloride (category Metal halides)
treated with ammonia, diverse ammine complexes are produced. In addition to the tetrahedral 1:2 complex ZnCl2(NH3)2. the complex Zn(NH3)4Cl2·H2O also has been...
39 KB (3,996 words) - 22:50, 20 November 2024
Copper(II) oxide (category Transition metal oxides)
ammonium carbonate, ammonia, and oxygen to ultimately give copper(II) ammine complex carbonates, such as [Cu(NH3)4]CO3. After extraction from the residues...
10 KB (829 words) - 15:20, 14 September 2024
Cisplatin (category Ammine complexes)
chemical name of this molecule is cis–diamminedichloroplatinum,: 286 where ammine with two m's indicates an ammonia (NH3) ligand, as opposed to an organic...
39 KB (3,864 words) - 22:43, 20 September 2024
Nitrate chlorides (redirect from Chlorido nitrato complex)
K. (1997-06-01). "Distortion of Crystal Structures of Some CoIII Ammine Complexes. I. Distortion of Crystal Structure of [Co(NH3)5NO2]Cl(NO3) on Cooling"...
63 KB (3,736 words) - 12:04, 2 November 2024
Silver bromide (category Metal halides)
bromide reacts readily with liquid ammonia to generate a variety of ammine complexes, like Ag(NH 3) 2Br and Ag(NH 3) 2Br− 2. In general: AgBr + m NH3 +...
18 KB (1,957 words) - 02:52, 31 October 2024