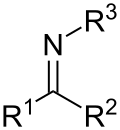
An oxaziridine is an organic molecule that features a three-membered heterocycle containing oxygen, nitrogen, and carbon. In their largest application...
26 KB (2,506 words) - 01:08, 14 September 2024
Davis reagent (redirect from Davis oxaziridine)
Davis reagent (3-phenyl-2-(phenylsulfonyl)-1,2-oxaziridine or 2-(benzenesulfonyl)-3-phenyloxaziridine) is a reagent used for oxidation in the Davis oxidation...
2 KB (122 words) - 12:15, 17 March 2022
the active dioxirane form before proceeding in the catalytic cycle. Oxaziridines such as chiral N-sulfonyloxaziridines effect enantioselective ketone...
19 KB (2,321 words) - 12:01, 2 March 2024
H2O Oxidation of the imine to the oxaziridine: Me(Et)C=NH + H2O2 → Me(Et)CONH + H2O Condensation of the oxaziridine with a second molecule of ammonia...
7 KB (780 words) - 12:31, 22 July 2023
deficient oxygen of the oxaziridine ring. This reaction type is extended to asymmetric synthesis by the use of chiral oxaziridines derived from camphor (camphorsulfonyl...
7 KB (651 words) - 16:56, 22 October 2024
In organic chemistry, the Davis oxidation or Davis' oxaziridine oxidation refers to oxidations involving the use of the Davis reagent...
6 KB (641 words) - 18:16, 26 September 2024
hydrogen peroxide to the oxaziridine, a three-membered ring containing carbon, oxygen, and nitrogen. Next, the oxaziridine gives the hydrazone by treatment...
51 KB (5,280 words) - 16:33, 31 October 2024
to: Formaldoxime Formamide, or methanamide Nitrosomethane [Wikidata] Oxaziridine This set index page lists chemical structure articles associated with...
271 bytes (60 words) - 18:06, 22 December 2022
reactions are useful for the enantioselective synthesis of chiral epoxides. Oxaziridine reagents may also be used to generate epoxides from alkenes. The Sharpless...
22 KB (2,324 words) - 21:50, 25 August 2024
74-88-4 CH3OCH3 dimethyl ether 115-10-6 CH3NH2 methylamine 74-89-5 CH3NO oxaziridine 6827-26-5 CH3OCs caesium methoxide CH3OH methanol 67-56-1 CH3OK potassium...
183 KB (107 words) - 22:11, 6 September 2024
Saturated Unsaturated 2 × Nitrogen Diaziridine Diazirine Nitrogen + Oxygen Oxaziridine Oxazirine Nitrogen + Sulfur Thiaziridine Thiazirine 2 × Oxygen Dioxirane...
20 KB (1,019 words) - 22:20, 25 October 2024
polymerization, Fe(acac)3 has been found to catalyze the reaction of N-sulfonyl oxaziridines with olefins to form 1,3-oxazolidine products. GHS: Sigma-Aldrich 517003...
6 KB (511 words) - 16:26, 24 October 2024
or sulfones; disulfides or thiols to sulfonyl halides; and imines to oxaziridines. It can also de-aromatize phenols. Heterogeneous reactions of sodium...
55 KB (5,828 words) - 23:02, 30 October 2024
reactivity may be reversed for some electron-deficient amines, including oxaziridines, hydroxylamines, oximes, and other N–O substrates. When the amine is...
3 KB (323 words) - 01:48, 12 October 2024
(10). Oxidation of the enolate of ketone 10 with (-)-camphorsulfonyl oxaziridine (11) gave α-hydroxyketone 12. Reduction of the ketone group with 20 equivalents...
16 KB (1,478 words) - 18:34, 27 September 2023
Imine are oxidized with meta-chloroperoxybenzoic acid (mCPBA) to give an oxaziridines. Imines are intermediates in the alkylation of amines with formic acid...
23 KB (2,633 words) - 01:56, 2 September 2024
for alpha-hydroxylation via enol or enolate structures include Davis oxaziridine, oxygen, and various peroxyacids (see Rubottom oxidation). This reagent...
7 KB (694 words) - 14:07, 14 July 2024
synthesis, Dioxiranes are intermediate in the Shi epoxidation reaction. Oxaziridine Ethylene oxide 1,2-Dioxetane 1,3-Dioxetane Lovas, F.J.; Suenram, R.D...
5 KB (446 words) - 09:42, 23 April 2024
tert-Butyl hydroperoxide or, more efficiently, (1S)-(+)-(10-camphorsulfonyl)-oxaziridine (CSO). The step of oxidation may be substituted with a sulfurization...
79 KB (9,460 words) - 05:24, 25 September 2024
organic oxidation with an electrophilic source of oxygen such as an oxaziridine or mCPBA. In the Saegusa–Ito oxidation, certain silyl enol ethers are...
13 KB (1,369 words) - 19:53, 12 February 2024
Johnson–Corey–Chaykovsky reaction and provides an alternative to amine transfer from oxaziridines. Though less widely applied, the reaction has a similar substrate scope...
22 KB (2,314 words) - 16:34, 26 September 2024
Wei; Carroll, P. J (1992). "Chemistry of Oxaziridines. 17. N-(Phenylsulfonyl)(3,3-dichlorocamphoryl)oxaziridine: A Highly Efficient Reagent for the Asymmetric...
15 KB (1,583 words) - 03:48, 22 October 2022
with peracids (such as cyclic sulfonimines as precursors of reactive oxaziridines) or sugar-based ketones that form bleach-active dioxiranes with hydrogen...
12 KB (1,430 words) - 09:30, 28 September 2024
Retrieved November 28, 2018. "CAREER: Catalyst-controlled Activation of Oxaziridines". nsf.gov. Retrieved November 28, 2018. "Tehshik Yoon". wisc.edu. Archived...
16 KB (1,458 words) - 03:22, 25 September 2024
including peroxides (often employed with a transition metal catalyst) and oxaziridines. These reagents do not suffer from the over-oxidation problems and decomposition...
11 KB (1,287 words) - 15:29, 1 February 2024
electron-withdrawing groups: chloramines, hydroxylamines, hydrazines, and oxaziridines, for instance. Addition reactions have employed imines, oximes, azides...
18 KB (2,059 words) - 01:08, 18 September 2024
compounds and synthesis via perfluorinated reagents (perfluorinated oxaziridines as powerful yet selective oxidizing agents) fluorinated contrast agents...
11 KB (1,077 words) - 05:02, 25 October 2024
oxidation was published by F.A. Davis in 1987 and showcased the Davis chiral oxaziridine methodology (Davis oxidation) to give good yields but modest enantiomeric...
28 KB (3,396 words) - 17:19, 26 September 2024
for oxidations of electron-poor double bonds, and sulfonyl-substituted oxaziridines are effective for electron-rich double bonds. Metal-based oxidants are...
9 KB (1,240 words) - 14:29, 8 June 2024
catalyst. The acyloin group in 13 was introduced by KHMDS and Davis’ oxaziridine (see Holton Taxol total synthesis for another use of this system) and...
9 KB (1,098 words) - 07:53, 11 June 2024