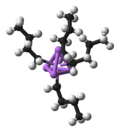
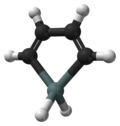
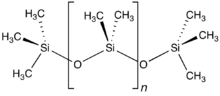
Organotin chemistry is the scientific study of the synthesis and properties of organotin compounds or stannanes, which are organometallic compounds containing...
24 KB (2,623 words) - 18:21, 19 September 2024
chemistry, organocadmium chemistry, organoindium chemistry, organotin chemistry, organoantimony chemistry, organotellurium chemistry Period 6 elements: organolanthanide...
31 KB (3,157 words) - 20:03, 24 September 2024
hexachlorostannic acid. Anhydrous tin(IV) chloride is a major precursor in organotin chemistry. Upon treatment with Grignard reagents, tin(IV) chloride gives tetraalkyltin...
9 KB (676 words) - 10:54, 14 July 2024
Heritage Stanthorpe Airport, IATA airport code "SNH" Organotin hydrides R4−nSnHn in organotin chemistry SNH48 This disambiguation page lists articles associated...
226 bytes (52 words) - 23:02, 22 March 2022
Tin (section Organotin compounds)
"Synthetic aspects of tetraorganotins and organotin(IV) halides" (PDF). Journal of Organometallic Chemistry. 689 (13): 2145–2157. doi:10.1016/j.jorganchem...
81 KB (8,852 words) - 23:42, 9 November 2024
Stannoxane (category Organotin compounds)
Stannoxane is a functional group in organotin chemistry with the connectivity SnIV−O−SnIV (IV indicates the oxidation state of tin). Aside from the oxide...
2 KB (200 words) - 18:36, 15 January 2023
Needlework Royal Saudi Navy RSN Racing & Sport RSn may refer to: Organotin chemistry and related compounds This disambiguation page lists articles associated...
499 bytes (89 words) - 04:25, 2 October 2023
slowly hydrolyze, slowly releasing toxins. These paints employed organotin chemistry ("tin-based") biotoxins such as tributyltin oxide (TBT) and were...
38 KB (4,410 words) - 19:32, 12 August 2024
and decreased fertility in Holtzmann rats. Davies, A. G. (2004). Organotin Chemistry. Weinheim, Germany: Wiley-VCH. ISBN 3-527-31023-1. G. G. Graf (2005)...
3 KB (144 words) - 15:57, 3 May 2023
Tributyltin oxide (category Organotin compounds)
(2004) Organotin Chemistry, 2nd Edition Weinheim: Wiley-VCH. ISBN 978-3-527-31023-4 National Pollutant Inventory Fact Sheet for organotins Tributyltin...
4 KB (171 words) - 06:30, 27 April 2024
Tributyltin (category Organotin compounds)
Tributyltin (TBT) is an umbrella term for a class of organotin compounds which contain the (C4H9)3Sn group, with a prominent example being tributyltin...
25 KB (3,039 words) - 15:24, 1 April 2024
Dibutyltin oxide (category Organotin compounds)
silicones and polyurethanes. Otera's catalyst Davies, Alwyn G. "Organotin Chemistry", 2nd Edition, 2004, Wiley-VCH: Weinheim. ISBN 978-3-527-31023-4...
4 KB (332 words) - 15:16, 1 June 2023
involves the coupling of two organic groups, one of which is carried as an organotin compound (also known as organostannanes). A variety of organic electrophiles...
47 KB (5,491 words) - 13:23, 22 February 2024
Dibutyltin dilaurate (category Organotin compounds)
Dibutyltin dilaurate (abbreviated DBTDL) is an organotin compound with the formula (CH3(CH2)10CO2)2Sn(CH2CH2CH2CH3)2. It is a colorless viscous and oily...
7 KB (437 words) - 11:51, 28 August 2024
was an English chemist. He was one of the originators of organometallic chemistry and introduced the concept of combining power or valence. An expert in...
24 KB (2,735 words) - 17:05, 26 February 2024
redistribution with silicon tetrachloride: SiMe4 + SiCl4 → 2 SiMe2Cl2 In organotin chemistry, the mixed alkyl tin chlorides are produced by redistribution, a...
3 KB (359 words) - 00:14, 3 November 2023
George Bowdler Buckton (section Chemistry)
ISSN 0276-7333. Nicholson, John W. (1989). "The early history of organotin chemistry". Journal of Chemical Education. 66 (8): 621. Bibcode:1989JChEd....
9 KB (886 words) - 16:29, 29 June 2024
cycloaddition reaction of two acetylene molecules with an organotin molecule SnR2. Organotin chemistry Dubac, Jacques; Laporterie, Andre; Manuel, Georges (1990)...
3 KB (245 words) - 20:32, 4 February 2024
in 1968. His research focused on organic peroxides, radicals and organotin chemistry. His students were sometimes referred to as "Alwyn's Tin Men". Davies...
5 KB (379 words) - 11:42, 5 September 2024
antioxidants; synthetic catalytic scavengers are their synthetic counterparts Organotin compounds are used in polymer manufacture as hydrochloric acid scavengers...
2 KB (254 words) - 17:36, 15 June 2022
(1991). "Structural chemistry of organotin carboxylates: a review of the crystallographic literature". Applied Organometallic Chemistry. 5: 1–23. doi:10...
4 KB (293 words) - 14:51, 3 January 2024
Heavy metal element (redirect from Heavy metal (Chemistry))
effects in fish, plants, birds, or other aquatic organisms); tin, as organotin (central nervous system damage); antimony (a suspected carcinogen); and...
171 KB (13,915 words) - 03:18, 10 November 2024
inorganic metal hydrides and group 14 hydrides. Organotin Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann...
3 KB (100 words) - 09:18, 12 April 2024
Tributyltin hydride (category Organotin compounds)
Tributyltin hydride is an organotin compound with the formula (C4H9)3SnH. It is a colorless liquid that is soluble in organic solvents. The compound is...
5 KB (425 words) - 22:34, 23 August 2024
nucleophiles and the reactivity of them is between that of organosilicon and organotin compounds. Some organogermanes have enhanced reactivity compared with...
9 KB (882 words) - 12:49, 1 May 2024
RTV silicone (section Chemistry)
Cervantes, Ramón Zárraga, Carmen Salazar-Hernández "Organotin catalysts in organosilicon chemistry" Appl. Organometal. Chem. 2012, vol. 26, 157–163. doi:10...
7 KB (826 words) - 16:55, 19 October 2024
chemical vapor deposition, vapour deposition techniques that employ SnCl4 or organotin trihalides e.g. butyltin trichloride as the volatile agent. This technique...
15 KB (1,500 words) - 02:45, 19 February 2024
Compounds of carbon with other group 14 elements: organogermanium compounds, organotin compounds, organolead compounds Silylenes, the carbene counterparts Silylenoids...
25 KB (2,547 words) - 00:08, 6 April 2024
Stannylene (category Organotin compounds)
Stannylenes (R2Sn:) are a class of organotin(II) compounds that are analogues of carbene. Unlike carbene, which usually has a triplet ground state, stannylenes...
13 KB (1,329 words) - 05:06, 8 February 2024
Triethylborane (section Organic chemistry)
effective even at low temperatures. As an initiator, it can replace some organotin compounds. It reacts with metal enolates, yielding enoxytriethylborates...
12 KB (1,020 words) - 11:19, 9 June 2024