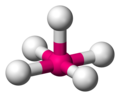
Valence shell electron pair repulsion (VSEPR) theory (/ˈvɛspər, vəˈsɛpər/ VESP-ər,: 410 və-SEP-ər) is a model used in chemistry to predict the geometry...
45 KB (4,041 words) - 16:04, 14 November 2024
levels of s and p orbitals. Bent's rule represents a modification of VSEPR theory for molecules of lower than ideal symmetry. For bonds with the larger...
38 KB (4,250 words) - 13:50, 4 October 2024
Chemical bond (redirect from Bonding theory)
polarity of bonds. The octet rule and VSEPR theory are examples. More sophisticated theories are valence bond theory, which includes orbital hybridization...
40 KB (4,872 words) - 13:33, 22 September 2024
pair is a concept used in valence shell electron pair repulsion theory (VSEPR theory) which explains the shapes of molecules. They are also referred to...
23 KB (2,957 words) - 14:53, 17 October 2024
Molecular geometry (section VSEPR table)
angles in the table below are ideal angles from the simple VSEPR theory (pronounced "Vesper Theory")[citation needed], followed by the actual angle for the...
23 KB (2,333 words) - 18:18, 20 November 2024
and methylene (CH2). This geometry is almost always consistent with VSEPR theory, which usually explains non-collinearity of atoms with a presence of...
3 KB (328 words) - 17:23, 26 January 2024
geometry are sometimes described as sp3 hybridized. The AXE method for VSEPR theory states that the classification is AX3E1. The nitrogen in ammonia has...
4 KB (318 words) - 16:20, 11 February 2024
above and below the plane (axial or apical positions). According to the VSEPR theory of molecular geometry, an axial position is more crowded because an axial...
6 KB (683 words) - 15:25, 7 February 2023
of steric activity is that of SnCl2, which is bent in accordance with VSEPR theory. Some examples where the lone pair appears to be inactive are bismuth(III)...
9 KB (1,155 words) - 01:57, 27 January 2024
Quantum chemistry (section Valence bond theory)
approaches are used, including semi-empirical methods, density functional theory, Hartree–Fock calculations, quantum Monte Carlo methods, and coupled cluster...
20 KB (2,232 words) - 18:50, 20 November 2024
1918 to the 1920s and from 1939 to 1945 Vespa Vespers (disambiguation) VSEPR theory in chemistry, often pronounced "Vesper" This disambiguation page lists...
3 KB (372 words) - 03:06, 2 August 2024
molecule is linear with a bond length consistent with strong ionic bonding. VSEPR theory would predict a bent shape similar to H 2O. Lithium oxide is used as...
7 KB (480 words) - 18:59, 8 March 2024
Orbital hybridisation (redirect from Hybridization theory)
repulsion (VSEPR) theory, which can be used to predict molecular geometry based on empirical rules rather than on valence-bond or orbital theories. As the...
33 KB (3,170 words) - 15:02, 7 November 2024
gas compound xenon tetrafluoride adopts this structure as predicted by VSEPR theory. The geometry is prevalent for transition metal complexes with d8 configuration...
5 KB (451 words) - 04:50, 4 November 2023
compounds that have a stereochemically-active lone pair, as described by VSEPR theory. Certain compounds crystallize in both the trigonal bipyramidal and the...
4 KB (303 words) - 22:44, 16 July 2023
pentagonal bipyramidal structure, with D5h symmetry, as predicted by VSEPR theory. The molecule can undergo a pseudorotational rearrangement called the...
8 KB (728 words) - 22:32, 5 April 2024
pentahalides have trigonal bipyramidal molecular geometry as explained by VSEPR theory. Phosphorus pentafluoride is a relatively inert gas, notable as a mild...
8 KB (426 words) - 21:39, 24 March 2024
precursor to acid rain. The molecule SO3 is trigonal planar. As predicted by VSEPR theory, its structure belongs to the D3h point group. The sulfur atom has an...
18 KB (1,557 words) - 20:11, 18 October 2024
a non-bonded lone pair on the sulfur, so the structure predicted by VSEPR theory is trigonal pyramidal, as in ammonia (NH3). In the hybrid resonance structure...
19 KB (1,745 words) - 14:49, 23 November 2024
T-shaped molecules are the halogen trifluorides, such as ClF3. According to VSEPR theory, T-shaped geometry results when three ligands and two lone pairs of electrons...
4 KB (377 words) - 08:02, 30 April 2024
qualitative theories. Such theories are easier to learn as they require little background in quantum theory. Within main group compounds, VSEPR theory powerfully...
29 KB (3,220 words) - 00:35, 17 November 2024
form. Wikimedia Commons has media related to Ball-and-stick models. VSEPR theory Turner M (1971). "Ball and stick models for organic chemistry". Journal...
5 KB (515 words) - 01:45, 1 October 2024
stereochemistry is denoted in skeletal formulae. Solid-state chemistry VSEPR theory Nuclear Overhauser effect, a method in nuclear magnetic resonance spectroscopy...
13 KB (1,461 words) - 11:17, 3 November 2024
the crystal, the species adopts a pyramidal structure, in accord with VSEPR theory. Bismuth chloride can be synthesized directly by passing chlorine over...
8 KB (592 words) - 18:43, 13 October 2022
this distortion is in pyramidal alkenes. AXE method Molecular geometry VSEPR theory March, Jerry (1985). Advanced organic chemistry : reactions, mechanisms...
3 KB (257 words) - 19:24, 26 January 2024
known as VSEPR theory. Examples of this structure include phosphorus pentafluoride and phosphorus pentachloride in the gaseous phase. In color theory, the...
17 KB (1,556 words) - 18:22, 18 November 2024
fourth apex of an approximately regular tetrahedron, as predicted by the VSEPR theory. This orbital is not participating in covalent bonding; it is electron-rich...
24 KB (2,751 words) - 20:34, 10 November 2024
should feature the strongest bonding of all group 17 monofluorides. VSEPR theory predicts a bent-T-shaped molecular geometry for the group 17 trifluorides...
69 KB (11,034 words) - 20:20, 25 November 2024
compounds, such as chloroform. Phosgene is a planar molecule as predicted by VSEPR theory. The C=O distance is 1.18 Å, the C−Cl distance is 1.74 Å and the Cl−C−Cl...
30 KB (3,132 words) - 00:07, 20 November 2024
is trigonal planar. Its D3h symmetry conforms with the prediction of VSEPR theory. The molecule has no dipole moment by virtue of its high symmetry. The...
19 KB (1,848 words) - 19:51, 29 October 2024