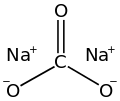
Calcium carbonate is a chemical compound with the chemical formula CaCO3. It is a common substance found in rocks as the minerals calcite and aragonite...
79 KB (7,544 words) - 06:17, 8 November 2024
compound; it exists only in aqueous solution containing calcium (Ca2+), bicarbonate (HCO− 3), and carbonate (CO2− 3) ions, together with dissolved carbon dioxide...
5 KB (373 words) - 15:44, 20 September 2023
mixtures of sodium carbonate, calcium carbonate, and silica sand (silicon dioxide (SiO2)). When these materials are heated, the carbonates release carbon...
32 KB (3,130 words) - 02:46, 8 November 2024
of calcium include calcium chloride and calcium gluconate. The forms that are taken by mouth include calcium acetate, calcium carbonate, calcium citrate...
38 KB (4,226 words) - 00:00, 18 November 2024
abundant metal, after iron and aluminium. The most common calcium compound on Earth is calcium carbonate, found in limestone and the fossilised remnants of early...
47 KB (5,906 words) - 06:50, 14 November 2024
dolomite, a calcium-magnesium carbonate CaMg(CO3)2; and siderite, or iron(II) carbonate, FeCO3, an important iron ore. Sodium carbonate ("soda" or "natron")...
18 KB (1,729 words) - 16:10, 19 November 2024
Limescale is a hard, chalky deposit, consisting mainly of calcium carbonate (CaCO3). It often builds up inside kettles, boilers, and pipework, especially...
9 KB (1,020 words) - 19:52, 9 July 2024
the most common carbonate rock and is a sedimentary rock made of calcium carbonate with two main polymorphs: calcite and aragonite. While the chemical...
13 KB (1,441 words) - 07:19, 16 November 2024
primary active component is calcium carbonate. Additional chemicals vary depending on the mineral source and may include calcium oxide. Unlike the types of...
13 KB (1,607 words) - 03:44, 24 June 2023
of limestone, chalk or gypsum, which are largely made up of calcium and magnesium carbonates, bicarbonates and sulfates. Drinking hard water may have moderate...
46 KB (4,637 words) - 07:42, 20 November 2024
Limestone (calcium carbonate CaCO3) is a type of carbonate sedimentary rock which is the main source of the material lime. It is composed mostly of the...
72 KB (8,485 words) - 15:26, 13 August 2024
(/kælˈkɛəriəs/) is an adjective meaning "mostly or partly composed of calcium carbonate", in other words, containing lime or being chalky. The term is used...
6 KB (654 words) - 17:33, 6 September 2024
The carbonate compensation depth (CCD) is the depth, in the oceans, at which the rate of supply of calcium carbonates matches the rate of solvation. That...
12 KB (1,369 words) - 13:34, 3 July 2024
Amorphous calcium carbonate (ACC) is the amorphous and least stable polymorph of calcium carbonate. ACC is extremely unstable under normal conditions and...
20 KB (2,194 words) - 10:48, 5 December 2023
broadly used term lime connotes calcium-containing inorganic compounds, in which carbonates, oxides, and hydroxides of calcium, silicon, magnesium, aluminium...
23 KB (2,231 words) - 10:54, 14 November 2024
Marine biogenic calcification is the production of calcium carbonate by organisms in the global ocean. Marine biogenic calcification is the biologically...
64 KB (7,212 words) - 19:35, 29 September 2024
Travertine (category Calcium minerals)
rusty varieties. It is formed by a process of rapid precipitation of calcium carbonate, often at the mouth of a hot spring or in a limestone cave. In the...
42 KB (4,554 words) - 00:56, 20 October 2024
(°dKH) (from the German "Karbonathärte"), or in parts per million calcium carbonate ( ppm CaCO 3 or grams CaCO 3 per litre|mg/L). One dKH is equal to...
3 KB (445 words) - 16:26, 17 March 2024
Calcite (category Carbonate minerals)
Calcite is a carbonate mineral and the most stable polymorph of calcium carbonate (CaCO3). It is a very common mineral, particularly as a component of...
46 KB (4,824 words) - 23:09, 21 October 2024
Lime (material) (category Calcium minerals)
primarily of calcium carbonate. They may be cut, crushed, or pulverized and chemically altered. Burning (calcination) of calcium carbonate in a lime kiln...
19 KB (2,351 words) - 19:28, 30 October 2024
Biological pump (redirect from Carbonate pump)
well as the cycling of calcium carbonate (CaCO3) formed into shells by certain organisms such as plankton and mollusks (carbonate pump). Budget calculations...
145 KB (16,326 words) - 13:47, 4 November 2024
driveways, are primarily made of gypsum rather than calcium carbonate chalk. Magnesium carbonate chalk is commonly used as a drying agent to obtain better...
23 KB (2,605 words) - 04:27, 31 October 2024
species have calcium carbonate exoskeletons. Calcareous sponges have calcium carbonate spicules and, in some species, calcium carbonate exoskeletons,...
132 KB (13,366 words) - 21:15, 29 October 2024
soils. Calcium ions are consumed and removed from aqueous environments as they react to form insoluble structures such as calcium carbonate and calcium silicate...
25 KB (2,993 words) - 18:54, 28 December 2023
Filler (materials) (section Calcium carbonate (CaCO3))
needs. The top filler materials used are ground calcium carbonate (GCC), precipitated calcium carbonate (PCC), kaolin, talc, and carbon black. Filler materials...
18 KB (2,133 words) - 09:20, 9 October 2024
24497 – Calcium Sulfate". PubChem. Titus, Harry W.; McNally, Edmund; Hilberg, Frank C. (1933-01-01). "Effect of Calcium Carbonate and Calcium Sulphate...
19 KB (1,836 words) - 04:41, 23 November 2024
very stable calcium silicate and releasing volatile (at high temperatures) magnesium metal. Via the carbonate–silicate cycle, carbonate rocks convert...
16 KB (1,394 words) - 12:01, 31 July 2024
Carbonatation is a chemical reaction in which calcium hydroxide reacts with carbon dioxide and forms insoluble calcium carbonate: Ca ( OH ) 2 + CO 2 ⟶ CaCO 3 + H 2...
6 KB (816 words) - 21:39, 7 November 2024
pregnancy and breastfeeding. Calcium gluconate is made by mixing gluconic acid with calcium carbonate or calcium hydroxide. Calcium gluconate came into medical...
19 KB (1,605 words) - 12:39, 20 November 2024
followed by reacting the sodium sulfate with coal and calcium carbonate to make sodium carbonate. The process gradually became obsolete after the development...
20 KB (2,541 words) - 04:45, 14 October 2024