In chemistry, a hypervalent molecule (the phenomenon is sometimes colloquially known as expanded octet) is a molecule that contains one or more main group...
39 KB (4,486 words) - 20:28, 22 July 2024
definitively excluding the role of d-orbital hybridisation in bonding in hypervalent compounds of second-row (period 3) elements, ending a point of contention...
33 KB (3,169 words) - 13:02, 22 September 2024
Octet rule (section Hypervalent molecules)
attain this configuration in compounds. There are, however, some hypervalent molecules in which the 3d level may play a part in the bonding, although this...
22 KB (2,869 words) - 13:52, 4 October 2024
Three-center four-electron bond (section Examples of molecules exhibiting three-center four-electron bonding)
4-electron (3c–4e) bond is a model used to explain bonding in certain hypervalent molecules such as tetratomic and hexatomic interhalogen compounds, sulfur...
11 KB (1,214 words) - 12:59, 22 September 2024
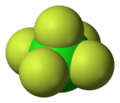
Sulfur hexafluoride (category Hypervalent molecules)
of six fluorine atoms attached to a central sulfur atom. It is a hypervalent molecule.[citation needed] Typical for a nonpolar gas, SF 6 is poorly soluble...
42 KB (4,064 words) - 02:28, 9 September 2024
Sulfur trioxide (category Hypervalent molecules)
violently with water to produce highly corrosive sulfuric acid. Hypervalent molecule Sulfur trioxide pyridine complex Greenwood, Norman N.; Earnshaw,...
18 KB (1,557 words) - 20:11, 18 October 2024
Resonance (chemistry) (section Aromatic molecules)
participation of d orbitals is unimportant, and the bonding of so-called hypervalent molecules are, for the most part, better explained by charge-separated contributing...
42 KB (5,094 words) - 06:04, 25 June 2024
Sulfur dioxide (category Hypervalent molecules)
known to medieval alchemists as "volatile spirit of sulfur". SO2 is a bent molecule with C2v symmetry point group. A valence bond theory approach considering...
53 KB (7,366 words) - 06:55, 17 September 2024
VALBOND (section Non-hypervalent molecules)
used by many force fields, and allows the VALBOND method to handle hypervalent molecules and transition metal complexes. The VALBOND energy term has been...
7 KB (1,245 words) - 18:52, 29 July 2024
Phosphorus pentachloride (category Hypervalent molecules)
Gaseous and molten PCl5 is a neutral molecule with trigonal bipyramidal geometry and (D3h) symmetry. The hypervalent nature of this species (as well as...
18 KB (1,562 words) - 08:25, 2 October 2024
fundamental contributions in polymer science. Beta-lactam Carbene Hypervalent molecule Polyoxymethylene Pyrethrin Triphenylphosphine phenylimide Heidegger...
17 KB (1,552 words) - 09:46, 20 August 2024
Triiodide (category Hypervalent molecules)
iodine-atom. In the molecular orbital model, a common explanation for the hypervalent bonding on the central iodine involves a three-center four-electron bond...
16 KB (1,587 words) - 04:56, 16 October 2024
Martin's sulfurane (category Hypervalent molecules)
solid that easily undergoes sublimation. The compound is an example of a hypervalent sulfur compound called a sulfurane. As such, the sulfur adopts a see-saw...
3 KB (165 words) - 12:24, 6 April 2023
types of organic synthesis as an efficient catalyst. Stannatrane Hypervalent molecule Voronkov, Mikhail G.; Baryshok, Viktor P. "Atranes - a new generation...
3 KB (328 words) - 01:19, 2 May 2022
of acetylacetone (2,4-pentanedione)). AXE method Square pyramid Hypervalent molecule Molecular geometry Spiro, Thomas G.; Terzis, Aristides; Raymond,...
4 KB (303 words) - 22:44, 16 July 2023
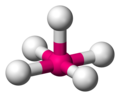
in the periodic table.) Lower-period elements, however, may form hypervalent molecules, such as phosphorus pentafluoride or sulfur hexafluoride. The reactivity...
85 KB (8,550 words) - 23:52, 26 August 2024
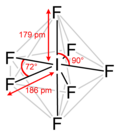
Sulfur tetrafluoride (category Hypervalent molecules)
3 pm and S–Feq = 154.2 pm. It is typical for the axial ligands in hypervalent molecules to be bonded less strongly. The 19F NMR spectrum of SF4 reveals...
10 KB (831 words) - 22:43, 29 September 2024
These are the hypervalent organoiodines, often called iodanes after the IUPAC rule used to name them. These iodine compounds are hypervalent because the...
18 KB (1,940 words) - 17:18, 30 June 2024
rearrangement 2,3-Wittig rearrangement Directed ortho metalation Ate complex Hypervalent molecule Potassium tetraphenylborate Awards Otto Hahn Prize for Chemistry...
7 KB (738 words) - 09:18, 20 August 2024
also be moved in the same way to create resonance structures for hypervalent molecules such as sulfur hexafluoride, which is the correct description according...
16 KB (2,140 words) - 19:28, 3 October 2024
electronegativity of the two bonded atoms. Pauling also considered hypervalent molecules, in which main-group elements have apparent valences greater than...
40 KB (2,914 words) - 06:38, 15 August 2024
in a large scale rocket propulsion system. Chlorine trifluoride Hypervalent molecule Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements...
6 KB (435 words) - 21:58, 26 August 2024
Fluorine (section Discrete molecules)
Noury, S.; Silvi, B.; Gillespie, R. J. (2002). "Chemical Bonding in Hypervalent Molecules: Is the Octet Rule Relevant?" (PDF). Inorganic Chemistry. 41 (8):...
157 KB (15,346 words) - 01:04, 8 September 2024
Tetraethylammonium trichloride (category Hypervalent molecules)
Tetraethylammonium trichloride (also known as Mioskowski reagent) is a chemical compound with the formula [NEt4][Cl3] consisting of a tetraethylammonium...
7 KB (612 words) - 04:31, 21 February 2023
Disulfur decafluoride (category Hypervalent molecules)
discovered in 1934 by Denbigh and Whytlaw-Gray. Each sulfur atom of the S2F10 molecule is octahedral, and surrounded by five fluorine atoms and one sulfur atom...
8 KB (654 words) - 01:34, 10 May 2024
Conjugated system (redirect from Conjugated molecule)
p-orbitals with delocalized electrons in a molecule, which in general lowers the overall energy of the molecule and increases stability. It is conventionally...
35 KB (4,303 words) - 03:15, 7 October 2024
than predicted by the octet rule, as explained in the article on hypervalent molecules. The mechanisms of their reactions differ from organic compounds...
29 KB (3,220 words) - 02:12, 22 August 2024
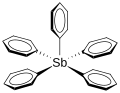
Pentaphenylantimony (category Hypervalent molecules)
phenyl groups all appear to be equivalent. This is probably because the molecule is not stable in shape and orientation of phenyl changes rapidly. Solid...
7 KB (721 words) - 22:03, 13 March 2024
Markku R. (1999). "Chemical Bonding in Hypervalent Molecules Revised. 2. Application of the Atoms in Molecules Theory to Y2XZ and Y2XZ2 (Y = H, F, CH3;...
20 KB (1,856 words) - 22:18, 15 April 2024
Iodine heptafluoride (category Hypervalent molecules)
bipyramidal structure, with D5h symmetry, as predicted by VSEPR theory. The molecule can undergo a pseudorotational rearrangement called the Bartell mechanism...
8 KB (728 words) - 22:32, 5 April 2024