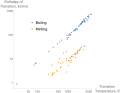
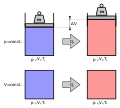
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change...
19 KB (2,776 words) - 19:20, 20 September 2024
In thermodynamics, the specific heat capacity (symbol c) of a substance is the amount of heat that must be added to one unit of mass of the substance...
55 KB (8,413 words) - 18:37, 15 October 2024
the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or Laplace's coefficient, is the ratio of the heat capacity at...
18 KB (2,318 words) - 16:04, 11 August 2024
The molar heat capacity of a chemical substance is the amount of energy that must be added, in the form of heat, to one mole of the substance in order...
50 KB (5,695 words) - 13:51, 6 October 2023
The table of specific heat capacities gives the volumetric heat capacity as well as the specific heat capacity of some substances and engineering materials...
15 KB (805 words) - 17:11, 1 September 2024
The volumetric heat capacity of a material is the heat capacity of a sample of the substance divided by the volume of the sample. It is the amount of energy...
21 KB (2,864 words) - 15:00, 20 May 2024
The heat capacity rate is heat transfer terminology used in thermodynamics and different forms of engineering denoting the quantity of heat a flowing...
5 KB (693 words) - 04:46, 4 July 2023
Einstein solid (redirect from Einstein's theory of specific heat capacity)
specific heat problem in classical mechanics. The original theory proposed by Einstein in 1907 has great historical relevance. The heat capacity of solids...
11 KB (1,962 words) - 05:07, 5 February 2024
capacity is the heat capacity per unit amount (SI unit: mole) of a pure substance, and the specific heat capacity, often called simply specific heat,...
90 KB (12,693 words) - 16:08, 20 October 2024
In thermodynamics, the heat capacity at constant volume, C V {\displaystyle C_{V}} , and the heat capacity at constant pressure, C P {\displaystyle C_{P}}...
10 KB (2,080 words) - 22:25, 31 August 2023
2003; Section 4, Properties of the Elements and Inorganic Compounds; Heat Capacity of the Elements at 25 °C As quoted at http://www.webelements.com/ from...
17 KB (209 words) - 17:51, 18 September 2024
Debye model (redirect from Debye theory of specific heat capacities)
phonon contribution to the specific heat (heat capacity) in a solid. It treats the vibrations of the atomic lattice (heat) as phonons in a box in contrast...
47 KB (9,148 words) - 14:58, 4 October 2024
This is a table of specific heat capacities by magnitude. Unless otherwise noted, these values assume standard ambient temperature and pressure....
3 KB (21 words) - 01:48, 21 May 2024
Isobaric process (section Specific heat capacity)
m}\end{aligned}}} where cP is molar heat capacity at a constant pressure. To find the molar specific heat capacity of the gas involved, the following equations...
12 KB (1,891 words) - 11:28, 12 May 2024
plant Heat capacity, a measurement of changes in a system's internal energy Combining capacity, another term for valence in chemistry Battery capacity, the...
2 KB (333 words) - 00:07, 21 May 2024
that temperature is low enough that heat capacities are not influenced by molecular vibration (see heat capacity). However, vibrational modes simply cause...
56 KB (7,935 words) - 21:40, 24 October 2024
refractive index specific conductance (or electrical conductivity) specific heat capacity, cp specific internal energy, u specific rotation, [α] specific volume...
20 KB (2,425 words) - 14:12, 15 September 2024
physics the electronic specific heat, sometimes called the electron heat capacity, is the specific heat of an electron gas. Heat is transported by phonons and...
17 KB (3,061 words) - 11:19, 1 February 2023
Ideal gas (section Heat capacity)
approximation). Since the heat capacity depends on the atomic or molecular nature of the gas, macroscopic measurements on heat capacity provide useful information...
24 KB (3,852 words) - 13:29, 20 September 2024
Free electron model (section Heat capacity)
and thermal conductivity; the temperature dependence of the electron heat capacity; the shape of the electronic density of states; the range of binding...
24 KB (3,404 words) - 13:09, 4 October 2024
can be any system with a non-zero heat capacity, but it usually is a gas or liquid. During this process, some heat is normally lost to the surroundings...
28 KB (3,858 words) - 15:55, 29 October 2024
Enthalpy of fusion (redirect from Latent heat of fusion)
processes independently and using the specific heat capacity of water to be 4.18 J/(g⋅K); thus, to heat 1 kg of ice from 273.15 K to water at 293.15 K...
12 KB (1,675 words) - 16:42, 14 September 2024
Properties of water (redirect from Heat capacity of water)
relatively high boiling point of 100 °C for its molar mass, and a high heat capacity. Water is amphoteric, meaning that it can exhibit properties of an acid...
89 KB (9,582 words) - 16:21, 28 October 2024
treated as lumped-capacitance heat reservoir, with total heat content which is proportional to simple total heat capacity C {\displaystyle C} , and T {\displaystyle...
26 KB (4,005 words) - 02:20, 1 June 2024
energies for a system at a given temperature, from which the system's heat capacity can be computed. However, equipartition also gives the average values...
90 KB (11,949 words) - 05:10, 19 June 2024
a function of time. The reference sample should have a well-defined heat capacity over the range of temperatures to be scanned. Additionally, the reference...
29 KB (3,627 words) - 02:37, 8 August 2024
Petit, states that the classical expression for the molar specific heat capacity of certain chemical elements is constant for temperatures far from the...
10 KB (1,510 words) - 14:19, 20 October 2024
Temperature (section Heat capacity)
Q}{\Delta T}}.} If heat capacity is measured for a well-defined amount of substance, the specific heat is the measure of the heat required to increase...
104 KB (12,956 words) - 19:12, 28 October 2024
{p}}-c_{\rm {v}}\ } where cp is the specific heat capacity for a constant pressure and cv is the specific heat capacity for a constant volume. It is common, especially...
17 KB (1,958 words) - 06:56, 21 October 2024
proportionality factor called the specific heat capacity of the material. By the combination of these observations, the heat equation says the rate u ˙ {\displaystyle...
58 KB (9,839 words) - 05:59, 23 October 2024