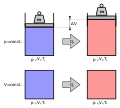
Absolute molar mass is a process used to determine the characteristics of molecules. The first absolute measurements of molecular weights (i.e. made without...
10 KB (1,534 words) - 11:02, 26 August 2022
mass and relative molecular mass are distinct from but related to the molar mass. The molar mass is defined as the mass of a given substance divided...
12 KB (1,482 words) - 17:11, 2 November 2024
In polymer chemistry, the molar mass distribution (or molecular weight distribution) describes the relationship between the number of moles of each polymer...
10 KB (1,391 words) - 12:22, 13 September 2024
Size-exclusion chromatography (redirect from Absolute size exclusion chromatography)
absolute molar mass and/or size measurements of proteins and macromolecules as they elute from the chromatography system. The definition of “absolute”...
31 KB (3,901 words) - 05:01, 30 October 2024
into a plurality of angles. It is used for determining both the absolute molar mass and the average size of molecules in solution, by detecting how they...
27 KB (3,599 words) - 12:58, 9 October 2024
times its molar mass. The SI unit of molar heat capacity is joule per kelvin per mole, J⋅K−1⋅mol−1. Like the specific heat, the measured molar heat capacity...
50 KB (5,696 words) - 21:09, 1 November 2024
Gas constant (redirect from Molar gas constant)
molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol R or R. It is the molar equivalent...
17 KB (1,958 words) - 06:56, 21 October 2024
experimentally determined molar mass of carbon-12. The relative isotopic mass (see section below) can be obtained by dividing the atomic mass ma of an isotope...
21 KB (2,721 words) - 03:04, 9 October 2024
light scattering analysis which allows the determination of the absolute molar mass. According to one study in the first step a DP of 265 is reached...
14 KB (1,500 words) - 19:51, 5 June 2024
Density of air (category Mass density)
{\displaystyle M} , molar mass R {\displaystyle R} , ideal gas constant T {\displaystyle T} , absolute temperature p {\displaystyle p} , absolute pressure Note...
17 KB (2,780 words) - 12:31, 4 October 2024
Dalton (unit) (redirect from Atomic mass units)
the mass of one mole of a substance in grams as numerically equal to the average mass of one of its particles in daltons. That is, the molar mass of a...
35 KB (3,683 words) - 19:30, 29 October 2024
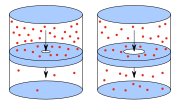
Diffusivity, mass diffusivity or diffusion coefficient is usually written as the proportionality constant between the molar flux due to molecular diffusion...
14 KB (1,630 words) - 16:19, 6 June 2024
Specific heat capacity (redirect from Molar specific heat)
or molar mass or a molar quantity is established, heat capacity as an intensive property can be expressed on a per mole basis instead of a per mass basis...
55 KB (8,416 words) - 13:24, 2 November 2024
De Laval nozzle (section Mass flow rate)
individual gas of molar mass M. The relationship between the two constants is Rs = R/M. In accordance with conservation of mass the mass flow rate of the...
15 KB (1,623 words) - 04:36, 6 October 2024
Standard cubic feet per minute (SCFM) is the molar flow rate of a gas expressed as a volumetric flow at a "standardized" temperature and pressure thus...
7 KB (1,020 words) - 10:29, 28 April 2024
Stoichiometry (redirect from Mass ratio (mixtures))
is numerically equal to the molar mass in g/mol. By definition, the atomic mass of carbon-12 is 12 Da, giving a molar mass of 12 g/mol. The number of molecules...
35 KB (5,274 words) - 20:42, 28 October 2024
the factor that converts the average mass ( m {\displaystyle m} ) of one particle, in grams, to the molar mass ( M {\displaystyle M} ) of the substance...
20 KB (2,232 words) - 23:10, 23 October 2024
concentrations from the molar to the mass concentration scale above are made as follows: Numerically: molar concentration × molar mass = mass concentration {\displaystyle...
108 KB (6,105 words) - 10:44, 2 July 2024
Equivalent weight (redirect from Equivalent mass)
used) are now derived from molar masses. The equivalent weight of a compound can also be calculated by dividing the molecular mass by the number of positive...
19 KB (2,706 words) - 02:54, 13 October 2024
List of measuring instruments (section Substance potential or chemical potential or molar Gibbs energy)
also Molar mass.) If specific molar values are given, then the amount of substance of a given sample may be determined by measuring volume, mass, or concentration...
45 KB (3,620 words) - 14:33, 29 August 2024
electrical potential mv (mass × velocity), momentum in physics Mv, viscosity average molar mass, a method to quantify molar mass distribution in chemistry...
3 KB (468 words) - 12:59, 16 October 2024
Thermodynamic temperature (redirect from Absolute temperature)
molecular mass of substance in kg/particle In the above formula, molecular mass, m, in kg/particle is the quotient of a substance's molar mass (also known...
105 KB (13,825 words) - 16:31, 18 October 2024
effusion of a gas is inversely proportional to the square root of the molar mass of its particles. This formula is stated as: Rate 1 Rate 2 = M 2 M 1 {\displaystyle...
8 KB (1,395 words) - 10:56, 1 November 2024
Yield (chemistry) (redirect from Molar yield)
0 mol ÷ 2.0 mol). The theoretical molar yield is 2.0 mol (the molar amount of the limiting compound, acetic acid). The molar yield of the product is calculated...
18 KB (2,205 words) - 01:04, 14 June 2024
Temperature (redirect from Absolute scale of temperature)
one of the seven base units in the International System of Units (SI). Absolute zero, i.e., zero kelvin or −273.15 °C, is the lowest point in the thermodynamic...
104 KB (12,956 words) - 19:12, 28 October 2024
the molar mass, N A {\displaystyle N_{\text{A}}} is the Avogadro constant, and R = N A k B {\displaystyle R=N_{\text{A}}k_{\text{B}}} is the molar gas...
8 KB (1,280 words) - 15:18, 2 November 2024
species to produce adducts rather than a protonated species. Mass spectrometry can measure molar mass, molecular structure, and sample purity. Each of these...
82 KB (10,326 words) - 02:15, 19 October 2024
ρ {\displaystyle \mu /\rho } is the mass absorption coefficient m a {\displaystyle m_{\text{a}}} is the molar mass in g/mol N A {\displaystyle N_{\text{A}}}...
4 KB (504 words) - 17:54, 10 January 2024
The standard liter per minute (SLM or SLPM) is a unit of (molar or) mass flow rate of a gas at standard conditions for temperature and pressure (STP),...
3 KB (560 words) - 04:32, 28 August 2023
Density (redirect from Mass density)
={\frac {MP}{RT}},} where M is the molar mass, P is the pressure, R is the universal gas constant, and T is the absolute temperature. This means that the...
35 KB (3,299 words) - 11:00, 29 October 2024