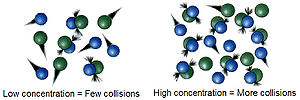
chemistry, the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based...
21 KB (2,776 words) - 19:56, 7 November 2024
significant global warming. The Arrhenius equation, Arrhenius acid, Arrhenius base, lunar crater Arrhenius, Martian crater Arrhenius, the mountain of Arrheniusfjellet...
38 KB (4,163 words) - 08:49, 13 September 2024
Arrhenius may refer to: Birgit Arrhenius (born 1932), Swedish archaeologist Carl Axel Arrhenius (1757–1824), Swedish army lieutenant and amateur mineralogist...
979 bytes (140 words) - 21:56, 4 October 2023
the Eyring equation is similar to the empirical Arrhenius equation, despite the Arrhenius equation being empirical and the Eyring equation based on statistical...
9 KB (1,077 words) - 16:14, 27 January 2024
Pre-exponential factor (redirect from Arrhenius factor)
pre-exponential factor or A factor is the pre-exponential constant in the Arrhenius equation (equation shown below), an empirical relationship between temperature and...
2 KB (281 words) - 04:32, 6 February 2024
Arrhenius plot gives a straight line, from which the activation energy and the pre-exponential factor can both be determined. The Arrhenius equation can...
6 KB (848 words) - 19:41, 8 April 2024
validity of the Arrhenius equation). At a more advanced level, the net Arrhenius activation energy term from the Arrhenius equation is best regarded...
19 KB (2,158 words) - 18:32, 6 November 2024
Clausius–Clapeyron relation Van 't Hoff factor (i) Gibbs–Helmholtz equation Solubility equilibrium Arrhenius equation Biography on Nobel prize website. Nobelprize.org (1...
22 KB (3,076 words) - 01:13, 10 August 2024
development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical...
41 KB (5,818 words) - 15:16, 21 October 2024
for E=0. Assume that the rate constants are well approximated by an Arrhenius equation, k f = A f exp [ − Δ ‡ G c / R T ] {\displaystyle k_{f}=A_{f}\exp[-\Delta...
18 KB (3,205 words) - 08:23, 20 July 2024
Functional equation Functional equation (L-function) Constitutive equation Laws of science Defining equation (physical chemistry) List of equations in classical...
5 KB (103 words) - 09:21, 8 August 2024
Reaction rate (section Rate equation)
rates can be independent of temperature (non-Arrhenius) or decrease with increasing temperature (anti-Arrhenius). Reactions without an activation barrier...
26 KB (3,716 words) - 19:46, 19 September 2024
in many fundamental equations in the physical sciences, such as the ideal gas law, the Arrhenius equation, and the Nernst equation. The gas constant is...
17 KB (1,958 words) - 06:56, 21 October 2024
application of the Arrhenius equation. The Evans–Polanyi model assumes that the pre-exponential factor of the Arrhenius equation and the position of...
3 KB (326 words) - 13:24, 31 July 2024
factor due to changes in temperature and is usually based on the Arrhenius equation. The total acceleration factor is the product of AFv and AFtemp The...
20 KB (2,726 words) - 20:45, 22 September 2024
this include (in addition to the results for ideal gases above) the Arrhenius equation in chemical kinetics. In statistical mechanics, the entropy S of an...
26 KB (2,916 words) - 20:59, 12 October 2024
Collision theory (section Rate equations)
expression is similar to the Arrhenius equation and gives the first theoretical explanation for the Arrhenius equation on a molecular basis. The weak...
22 KB (3,224 words) - 05:09, 30 September 2024
Thermionic emission (redirect from Richardson-Dushman equation)
temperature of the wire with a mathematical form similar to the modified Arrhenius equation, T 1 / 2 e − b / T {\displaystyle T^{1/2}\mathrm {e} ^{-b/T}} . Later...
32 KB (3,564 words) - 16:29, 25 October 2024
viscosity[clarification needed]. It is also called Néel-Arrhenius theory, after the Arrhenius equation, and Néel-Brown theory after a more rigorous derivation...
5 KB (668 words) - 16:55, 29 September 2023
Marcus theory (redirect from Marcus equation)
{\displaystyle \lambda =\lambda _{\text{in}}+\lambda _{o}} and inserted in the Arrhenius equation k act = A ⋅ e − Δ G in ‡ + Δ G o ‡ k T {\displaystyle k_{\text{act}}=A\cdot...
40 KB (5,759 words) - 12:52, 4 October 2024
for a reaction is experimentally determined through the Arrhenius equation and the Eyring equation. The main factors that influence the reaction rate include:...
24 KB (3,326 words) - 19:09, 2 November 2024
using the Arrhenius equation and a realistic relative humidity. Accelerated aging techniques, particularly those using the Arrhenius equation, have frequently...
11 KB (1,469 words) - 03:19, 15 December 2023
exponential dependence of a reaction rate on temperature is known as the Arrhenius equation. The activation energy necessary for a chemical reaction can be provided...
59 KB (7,393 words) - 20:56, 26 October 2024
determine the activation energy E A {\displaystyle E_{A}} , using the Arrhenius equation: σ = σ 0 e − E a / R T {\displaystyle \sigma =\sigma _{0}e^{-E_{a}/RT}}...
30 KB (3,493 words) - 02:21, 23 April 2024
called the Néel relaxation time and is given by the following Néel–Arrhenius equation: τ − 1 = τ 0 − 1 exp ( − U e f f k B T ) {\displaystyle \tau ^{-1}=\tau...
30 KB (3,168 words) - 15:42, 9 January 2024
the Arrhenius equation (2) through modification of the activation energy for viscous flow. At the same time equilibrium liquids follow the Arrhenius equation...
99 KB (11,449 words) - 16:31, 25 October 2024
but on the ratio of that energy and kT, that is, on E/kT (see Arrhenius equation, Boltzmann factor). For a system in equilibrium in canonical ensemble...
3 KB (311 words) - 20:08, 4 October 2024
This is a list of scientific equations named after people (eponymous equations). Contents A B C D E F G H I J K L M N O P R S T V W Y Z See also References...
30 KB (438 words) - 21:15, 3 October 2024
of the metal. The creation of lattice vacancies is governed by the Arrhenius equation, and the migration/diffusion of lattice vacancies are governed by...
16 KB (2,090 words) - 16:41, 17 September 2024
Chemical reaction (section Equations)
The temperature dependence of the rate constant usually follows the Arrhenius equation: k = k 0 e − E a / k B T {\displaystyle k=k_{0}e^{{-E_{a}}/{k_{B}T}}}...
66 KB (8,043 words) - 01:08, 14 October 2024