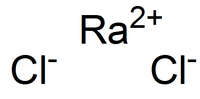Barium chloride is an inorganic compound with the formula BaCl2. It is one of the most common water-soluble salts of barium. Like most other water-soluble...
13 KB (1,067 words) - 16:01, 14 October 2024
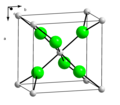
from barium. The first preparation of radium metal was by the electrolysis of a solution of this salt using a mercury cathode. Radium chloride crystallises...
7 KB (705 words) - 15:10, 25 July 2024
: 12–13 Barium, typically as barium nitrate imparts a yellow or "apple" green color to fireworks; for brilliant green barium chloride is used. Barium peroxide...
32 KB (3,763 words) - 17:08, 3 October 2024
are intermediate between those for barium chloride, which is more toxic, and calcium chloride. Strontium chloride can be prepared by treating aqueous...
11 KB (923 words) - 20:03, 20 August 2024
Barium chloride fluoride is an inorganic chemical compound of barium, chlorine, and fluorine. Its chemical formula is BaClF. The compound naturally occurs...
4 KB (303 words) - 17:43, 29 April 2024
Barium bromide is the chemical compound with the formula BaBr2. It is ionic and hygroscopic in nature. BaBr2 crystallizes in the lead chloride (cotunnite)...
8 KB (426 words) - 01:16, 15 October 2024
Magnesium sulfate is used to treat barium chloride poisoning, where sulfate binds to barium to form insoluble barium sulfate. Magnesium sulfate was historically...
24 KB (2,153 words) - 06:22, 19 October 2024
made by mixing specified amounts of barium chloride and sulfuric acid together. Mixing the two compounds forms a barium sulfate precipitate, which causes...
3 KB (307 words) - 14:49, 29 April 2023
to form soluble barium salts, such as barium chloride: BaCO3 + 2 HCl → BaCl2 + CO2 + H2O Pyrolysis of barium carbonate gives barium oxide. It is mainly...
6 KB (413 words) - 03:54, 27 May 2024
Arsine – AsH3 Barium azide – Ba(N3)2 Barium bromide – BaBr2 Barium carbonate – BaCO3 Barium chlorate – Ba(ClO3)2 Barium chloride – BaCl2 Barium chromate –...
119 KB (8,735 words) - 14:26, 16 September 2024
This page provides supplementary chemical data on barium chloride. SIRI Science Stuff (Dihydrate) CRC handbook of chemistry and physics : a ready-reference...
5 KB (132 words) - 14:55, 11 April 2023
Radium (redirect from Eka-barium)
Small amounts of barium impurities give the compound a rose color. Its It is soluble in water, though less so than barium chloride, and its solubility...
75 KB (8,080 words) - 19:22, 18 October 2024
Magnesium chloride is an inorganic compound with the formula MgCl2. It forms hydrates MgCl2·nH2O, where n can range from 1 to 12. These salts are colorless...
23 KB (2,183 words) - 11:55, 15 October 2024
Brackett, T. E.; Sass, R. L.; The Crystal Structures of Barium Chloride, Barium Bromide, and Barium Iodide. J. Phys. Chem., 1963, volume 67, 2132 – 2135...
5 KB (275 words) - 00:13, 18 July 2023
hydrochloric acid and nitric acid give the sulfate, chloride and nitrate respectively.[citation needed] Barium acetate is used as a mordant for printing textile...
5 KB (355 words) - 05:01, 23 September 2024
Reactions of barium hydroxide with ammonium salts are strongly endothermic. The reaction of barium hydroxide octahydrate with ammonium chloride or ammonium...
11 KB (927 words) - 01:27, 6 October 2024
from fluxed melt of barium borate, sodium oxide and sodium chloride. Thin films of barium borate can be prepared by MOCVD from barium(II) hydro-tri(1-pyrazolyl)borate...
13 KB (1,321 words) - 14:40, 5 May 2024
crystals of barium ferrite, by mixing barium chloride, ferrous chloride, potassium nitrate, and sodium hydroxide with a hydroxide to chloride concentration...
16 KB (1,562 words) - 23:00, 12 January 2024
production of chloric acid. Barium chlorate can be produced through a double replacement reaction between solutions of barium chloride and sodium chlorate: BaCl2...
6 KB (508 words) - 18:03, 25 September 2023
analog of baryte, BaSO4. It can be synthesized by reacting barium hydroxide or barium chloride with potassium chromate. Ba ( OH ) 2 + K 2 CrO 4 ⟶ BaCrO...
9 KB (1,049 words) - 14:24, 10 January 2024
donors) are frequently added too, the most common of which is polyvinyl chloride. A practical use of colored fire is the flame test, where metal cations...
6 KB (472 words) - 18:23, 13 August 2024
nitrite. Barium nitrite can be made by reacting barium nitrate with lead metal sponge, or by reaction of lead nitrite with barium chloride. Barium nitrite...
2 KB (89 words) - 19:51, 4 January 2024
administration of magnesium sulfate in barium chloride poisoning, where sulfate binds to barium to form insoluble barium sulfate. Magnesium is absorbed orally...
13 KB (969 words) - 05:23, 6 September 2024
Barium sulfate (or sulphate) is the inorganic compound with the chemical formula BaSO4. It is a white crystalline solid that is odorless and insoluble...
18 KB (2,026 words) - 23:28, 19 August 2024
Alkaline earth metal (section Barium)
as calcium chloride (CaCl 2), as well as reacting with oxygen to form oxides such as strontium oxide (SrO). Calcium, strontium, and barium react with...
71 KB (7,264 words) - 13:43, 4 October 2024
caused by a chemical reaction. When a barium chloride solution reacts with sulphuric acid, a white precipitate of barium sulphate is formed. When a potassium...
12 KB (1,330 words) - 03:30, 22 September 2024
Calcium chloride is an inorganic compound, a salt with the chemical formula CaCl2. It is a white crystalline solid at room temperature, and it is highly...
36 KB (2,912 words) - 11:52, 15 October 2024
reaction of sulfur with fuming nitric acid forms insoluble barium sulfate on addition of barium chloride. The purpose of adding the nitric acid is to oxidise...
1 KB (190 words) - 13:44, 9 January 2023
solution and a barium chloride solution, with the reaction as follows: BaCl2 + H2C2O4 → BaC2O4↓ + 2 HCl rec.pyrotechnics FAQ MSDS Barium Oxalate ChemSpider...
3 KB (196 words) - 06:03, 25 December 2023
Beryllium chloride is an inorganic compound with the formula BeCl2. It is a colourless, hygroscopic solid that dissolves well in many polar solvents. Its...
8 KB (570 words) - 22:15, 10 August 2024