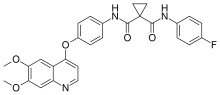
Cabozantinib, sold under the brand names Cometriq and Cabometyx among others, is an anti-cancer medication used to treat medullary thyroid cancer, renal...
21 KB (1,640 words) - 06:51, 18 September 2024
partnered its lead cancer drug candidate, XL-184 (which would become called cabozantinib) and another cancer candidate, XL-281, with Bristol Myers Squibb; BMS...
12 KB (1,034 words) - 18:16, 11 July 2024
surgery. Cabozantinib, trade name Cometriq, was granted marketing approval (November 2012) by the U.S. FDA for this indication. Cabozantinib which is...
19 KB (2,285 words) - 11:17, 14 October 2024
daclatasvir, sofosbuvir, afatinib, axitinib, brigatinib, baricitinib, cabozantinib, dasatinib, neratinib, eltrombopag, ibrutinib, lenvatinib, palbociclib...
5 KB (232 words) - 10:23, 8 March 2024
common adverse reaction to cytotoxic chemotherapy drugs, particularly cabozantinib, cytarabine, doxorubicin, and fluorouracil and its prodrug capecitabine...
16 KB (1,638 words) - 17:34, 11 November 2024
Institute. His work has led to the establishment of several novel drugs (cabozantinib, avelumab, pazopanib and others including combinations) and prognostic...
17 KB (1,497 words) - 05:06, 18 December 2024
that it is due to the decreased absorption of abacavir by orlistat. Cabozantinib: Drugs from the MRP2 inhibitor (Multidrug resistance-associated protein...
32 KB (2,750 words) - 08:14, 30 November 2024
that possess a broad range of targets along with RET. These included cabozantinib, lenvatinib, sunitinib and alectinib. Since they were not designed to...
20 KB (2,041 words) - 22:01, 24 November 2024
ipilimumab combination therapy, or in combination with chemotherapy or cabozantinib. The approval includes indications for renal cell carcinoma, melanoma...
7 KB (364 words) - 07:33, 28 December 2024
(PNB-0408) Kinase inhibitors: Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a...
121 KB (13,818 words) - 07:04, 26 December 2024
cell carcinoma over everolimus in 2015 and was approved by the FDA. Cabozantinib also demonstrated an overall survival benefit over everolimus and was...
56 KB (5,187 words) - 00:00, 31 December 2024
cancer (MTC) who require systemic therapy following prior treatment with cabozantinib and/or vandetanib. In September 2024, the FDA granted traditional approval...
26 KB (2,263 words) - 08:50, 31 December 2024
(PNB-0408) Kinase inhibitors: Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a...
149 KB (15,719 words) - 16:46, 9 December 2024
(PNB-0408) Kinase inhibitors: Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a...
73 KB (6,489 words) - 08:56, 21 December 2024
cabergoline (INN) cabiotraxetan (INN) Cablivi Cabometyx cabotegravir cabozantinib (USAN, INN) Cabtreo cactinomycin (INN) cadazolid (INN) cadexomer (INN)...
16 KB (757 words) - 05:06, 19 June 2024
vascular endothelial growth factor receptor 2 (VEGFR-2). c-Met inhibitors Cabozantinib, a similar molecule and kinase inhibitor with FDA approval VEGFR inhibitor...
4 KB (183 words) - 09:39, 5 September 2024
(PNB-0408) Kinase inhibitors: Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a...
11 KB (666 words) - 00:54, 27 December 2024
(PNB-0408) Kinase inhibitors: Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a...
13 KB (1,177 words) - 01:44, 4 November 2024
(PNB-0408) Kinase inhibitors: Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a...
93 KB (8,733 words) - 03:33, 16 December 2024
c-Met inhibitors are currently[when?] in clinical trials. Crizotinib and cabozantinib were the first to be approved by the U.S. FDA. Crizotinib received accelerated...
32 KB (3,542 words) - 19:34, 2 July 2024
Clinical trial number NCT03141177 for "A Study of Nivolumab Combined With Cabozantinib Compared to Sunitinib in Previously Untreated Advanced or Metastatic...
46 KB (4,813 words) - 05:44, 28 December 2024
spasticity in children aged two years and older. In 2016, Ipsen licensed cabozantinib from Exelixis, which received marketing authorization the same year for...
23 KB (2,392 words) - 09:39, 10 December 2024
(PNB-0408) Kinase inhibitors: Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a...
25 KB (1,825 words) - 05:46, 16 December 2024
(PNB-0408) Kinase inhibitors: Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a...
109 KB (10,674 words) - 22:46, 10 December 2024
(PNB-0408) Kinase inhibitors: Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a...
26 KB (2,420 words) - 02:40, 26 September 2024
(PNB-0408) Kinase inhibitors: Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a...
64 KB (7,432 words) - 08:21, 28 October 2024
(PNB-0408) Kinase inhibitors: Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a...
22 KB (1,994 words) - 08:45, 4 November 2024
those who had tumor progression while on sorafenib compared to placebo. Cabozantinib, which is an inhibitor of multiple tyrosine kinases including VEGFR,...
91 KB (10,155 words) - 06:43, 30 December 2024
(PNB-0408) Kinase inhibitors: Altiratinib AM7 AMG-458 Amuvatinib BMS-777607 Cabozantinib Capmatinib Crizotinib Foretinib Golvatinib INCB28060 JNJ-38877605 K252a...
11 KB (936 words) - 20:27, 30 August 2024
Pazopanib L01EX04 Vandetanib L01EX05 Regorafenib L01EX06 Masitinib L01EX07 Cabozantinib L01EX08 Lenvatinib L01EX09 Nintedanib L01EX10 Midostaurin L01EX11 Quizartinib...
13 KB (935 words) - 04:36, 23 December 2024