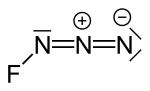Chlorine azide (ClN3) is an inorganic compound that was discovered in 1908 by Friedrich Raschig. Concentrated ClN3 is notoriously unstable and may spontaneously...
4 KB (262 words) - 00:31, 4 December 2023
ligands attached than the original chlorine ligands. Converting a ligand with N–N bonds, such as hydrazine or azide, directly into a dinitrogen ligand...
34 KB (4,298 words) - 07:31, 17 May 2024
chlorine thiocyanate (ClSCN, unlike its oxygen counterpart), and chlorine azide (ClN3). Chlorine monofluoride (ClF) is extremely thermally stable, and is sold...
117 KB (13,043 words) - 00:36, 25 September 2024
school chemistry students or as an act of "chemical magic". Chlorine azide (ClN3) and bromine azide (BrN3) are extremely sensitive and explosive. Two series...
105 KB (12,206 words) - 12:19, 27 September 2024
2003 by N. Hansen and A. M. Wodtke using ultraviolet photolysis of chlorine azide. Although the reaction yielded mostly the linear form, about 20% of...
4 KB (387 words) - 11:35, 28 June 2024
sensitive. Many inorganic covalent azides (e.g., chlorine, bromine, and iodine azides) have been described. The azide anion behaves as a nucleophile; it...
19 KB (2,184 words) - 15:14, 27 September 2024
Shock sensitivity Frierson, W. Joe, J. Kronrad, and A. W. Browne. "Chlorine Azide, CIN3. I1." - Journal of the American Chemical Society (ACS Publications)...
9 KB (1,058 words) - 20:29, 1 September 2024
azide (IN3) is an explosive inorganic compound, which in ordinary conditions is a yellow solid. Formally, it is an inter-pseudohalogen. Iodine azide can...
7 KB (579 words) - 15:43, 22 August 2024
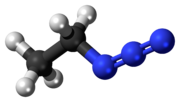
Ethyl azide (CH3CH2N3) is an explosive compound sensitive to rapid heating, shock or impact. It has exploded when heated to room temperature. When heated...
3 KB (183 words) - 10:41, 13 August 2023
Methyl azide is an organic compound with the formula CH3N3. It is a white solid and it is the simplest organic azide. Methyl azide can be prepared by...
5 KB (394 words) - 09:05, 2 September 2024
(Cl−NO) and chlorine azide (Cl−N3), as well as interpseudohalogens like dinitrogen trioxide (O=N−NO2), nitric acid (HO−NO2) and cyanogen azide (N3−CN). Not...
10 KB (566 words) - 22:03, 24 August 2024
dinitride Azides: Cyanuric triazide, Cyanogen azide, Chlorine azide, Copper(II) azide, Ethyl azide, Fluorine azide, Hydrazoic azide, Lead(II) azide, Silver...
72 KB (8,223 words) - 00:30, 17 September 2024
Ammonium azide is the chemical compound with the formula [NH4]N3, being the salt of ammonia and hydrazoic acid. Like other inorganic azides, this colourless...
5 KB (244 words) - 14:03, 3 August 2024
Bromine azide is an explosive inorganic compound with the formula BrN3. It has been described as a crystal or a red liquid at room temperature.[citation...
6 KB (523 words) - 14:43, 13 December 2023
Fluorine azide or triazadienyl fluoride is a yellow green gas composed of nitrogen and fluorine with formula FN3. Its properties resemble those of ClN3...
8 KB (728 words) - 22:43, 21 July 2024
Hydrazoic acid (redirect from Hydrogen azide)
Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, is a compound with the chemical formula HN3. It is a colorless, volatile, and explosive...
12 KB (1,139 words) - 14:08, 30 December 2023
acid – HClO3 Chlorine azide – ClN3 Chlorine dioxide – ClO2 Chlorine dioxide – ClO2 Chlorine monofluoride – ClF Chlorine monoxide – ClO Chlorine pentafluoride...
119 KB (8,735 words) - 14:26, 16 September 2024
n-Propyl azide is an organic compound with the formula CH3CH2CH2N3. A white solid, it is a simple organic azide. n-Propyl azide has been used in the laboratory...
3 KB (164 words) - 07:36, 19 July 2023
similarly coloured vapour. Its properties are intermediate between those of chlorine and iodine. Isolated independently by two chemists, Carl Jacob Löwig (in...
67 KB (7,693 words) - 20:33, 10 August 2024
temperature. Because of the difference in the electronegativity of iodine and chlorine, this molecule is highly polar and behaves as a source of I+. Discovered...
6 KB (478 words) - 07:22, 4 December 2023
Organochlorine chemistry (section From chlorine)
one covalently bonded atom of chlorine. The chloroalkane class (alkanes with one or more hydrogens substituted by chlorine) includes common examples. The...
17 KB (1,912 words) - 13:30, 25 September 2024
Azinamine (category Azides)
(N10). Frierson, W. Joe; Kronrad, J.; Browne, A. W. (September 1943). "Chlorine Azide, ClN3 I". Journal of the American Chemical Society. 65 (9): 1696–1698...
806 bytes (82 words) - 16:47, 17 May 2024
compounds in which the only anion or ligand is the azide group, -N3. The breadth of homoleptic azide compounds spans nearly the entire periodic table....
71 KB (7,995 words) - 14:43, 9 September 2024
chlorimide can be made by chlorinating vanadium nitride at 120°. Or chlorine azide can react with vanadium tetrachloride to yield it. Or more conveniently...
6 KB (602 words) - 09:06, 3 January 2024
+3 respectively: 2 NO2 + H2O → HNO3 + HNO2 In hydrazoic acid and sodium azide, each of the 3 nitrogen atoms of these very energetic linear polyatomic...
17 KB (1,897 words) - 07:47, 22 August 2024
following values for typical nucleophilic anions: acetate 2.7, chloride 3.0, azide 4.0, hydroxide 4.2, aniline 4.5, iodide 5.0, and thiosulfate 6.4. Typical...
17 KB (2,157 words) - 13:02, 11 September 2024
fulminate alone. Silver fulminate is often confused with silver nitride, silver azide, or fulminating silver. "Fulminating silver", though always referring to...
9 KB (948 words) - 15:35, 23 January 2024
chlorine are more electrophilic and are more aggressive halogenating agents. Bromine is a weaker halogenating agent than both fluorine and chlorine,...
10 KB (1,112 words) - 15:24, 12 June 2024
known, such as cyanogen iodide (ICN), iodine thiocyanate (ISCN), and iodine azide (IN3). Iodine monofluoride (IF) is unstable at room temperature and disproportionates...
107 KB (11,980 words) - 14:46, 27 September 2024
hemoglobin and certain cytochromes in a manner analogous to cyanide and azide. The two principal sulfur oxides are obtained by burning sulfur: S + O2...
10 KB (1,150 words) - 04:23, 9 February 2024