Cobalt(II) carbonate is the inorganic compound with the formula CoCO3. This pink paramagnetic solid is an intermediate in the hydrometallurgical purification...
8 KB (547 words) - 15:02, 6 September 2024
mutually trans aquo ligands. Cobalt chloride can be prepared in aqueous solution from cobalt(II) hydroxide or cobalt(II) carbonate and hydrochloric acid: CoCO...
21 KB (2,143 words) - 12:50, 13 October 2024
(1986). "Structures of hydrothermally synthesized cobalt(II) carbonate and nickel(II) carbonate". Acta Crystallographica Section C. 42 (1): 4–5. Bibcode:1986AcCrC...
6 KB (533 words) - 22:13, 30 October 2024
(1986). "Structures of hydrothermally synthesized cobalt(II) carbonate and nickel(II) carbonate". Acta Crystallographica Section C. 42: 4–5. doi:10...
5 KB (284 words) - 11:25, 12 January 2023
are both hygroscopic solids. Cobalt(II) perchlorate hexahydrate is produced by reacting cobalt metal or cobalt(II) carbonate with perchloric acid, followed...
6 KB (399 words) - 11:05, 12 May 2024
Cobalt(II) oxide is prepared by oxidation of cobalt powder with air or by thermal decomposition of cobalt(II) nitrate or the carbonate. Cobalt(II,III)...
5 KB (417 words) - 11:17, 5 March 2024
methods and from which cobalt can be recovered by electro refining or by carbonate precipitation. If hydrochloric acid is used then cobalt may be extracted...
8 KB (1,079 words) - 23:21, 9 February 2024
Cobalt(II) hydroxide or cobaltous hydroxide is the inorganic compound with the formula Co(OH) 2, consisting of divalent cobalt cations Co2+ and hydroxide...
9 KB (860 words) - 21:06, 17 July 2024
Cobalt nitrate is the inorganic compound with the formula Co(NO3)2.xH2O. It is cobalt(II)'s salt. The most common form is the hexahydrate Co(NO3)2·6H2O...
8 KB (580 words) - 01:27, 8 October 2024
Cobalt(II) sulfate is any of the inorganic compounds with the formula CoSO4(H2O)x. Usually cobalt sulfate refers to the hexa- or heptahydrates CoSO4.6H2O...
10 KB (735 words) - 20:16, 10 September 2024
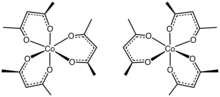
individual enantiomers. Tris(acetylacetonato)cobalt(III) is prepared by the reaction of cobalt(II) carbonate and acetylacetone in the presence of hydrogen...
4 KB (305 words) - 10:39, 1 March 2024
cobalt carbonate, in a glass melt. Cobalt is a very intense colouring agent and very little is required to show a noticeable amount of colour. Cobalt...
10 KB (1,124 words) - 21:48, 24 July 2024
Cobalt(II) bromide – CoBr2 Cobalt(II) carbonate – CoCO3 Cobalt(II) chloride – CoCl2 Cobalt(II) nitrate – Co(NO3)2 Cobalt(II) sulfate – CoSO4 Cobalt(III)...
119 KB (8,735 words) - 17:31, 25 October 2024
Coco3 can refer to: Cobalt(II) carbonate, an inorganic compound with the formula CoCO3 The third and final version of the TRS-80 Color Computer, a 6809-based...
253 bytes (71 words) - 01:16, 23 November 2017
dihalides of cobalt(II) are known: cobalt(II) fluoride (CoF2, pink), cobalt(II) chloride (CoCl2, blue), cobalt(II) bromide (CoBr2, green), cobalt(II) iodide...
116 KB (11,882 words) - 02:46, 30 October 2024
peroxide to a solution of cobalt(II) chloride in 96% ethanol at –30 to –35°C, then adding a 15% solution of sodium carbonate in water with intense stirring...
4 KB (255 words) - 22:03, 7 November 2023
structures, the most popular of which are lithium cobalt oxide and lithium iron phosphate. In 1843, lithium carbonate was used to treat stones in the bladder....
30 KB (2,641 words) - 01:21, 30 October 2024
lithium cobalt oxide can be prepared by heating a stoichiometric mixture of lithium carbonate Li 2CO 3 and cobalt(II,III) oxide Co 3O 4 or metallic cobalt at...
12 KB (1,260 words) - 17:28, 4 July 2024
both bi- and unidentate carbonate ligands. The addition of [Co(NH3)6]Cl3 to fresh solutions of sodium tris(carbonato)cobalt(III) precipitates anhydrous...
7 KB (643 words) - 14:27, 30 May 2024
Cobalt compounds are chemical compounds formed by cobalt with other elements. Many halides of cobalt(II) are known.e cobalt(II) fluoride (CoF2) which is...
16 KB (1,838 words) - 16:18, 6 September 2024
Chalk – a rock composed of porous biogenic calcium carbonate. CaCO3 Chrome green – chromic oxide and cobalt oxide. Chrome orange – chrome yellow and chrome...
14 KB (1,523 words) - 06:19, 24 October 2024
8(AlSiO 4) 6(S,SO 4,Cl) 1–2. Cobalt pigments Cobalt blue (PB28): cobalt(II) aluminate. Cerulean blue (PB35): cobalt(II) stannate. Cerium uranium blue...
9 KB (909 words) - 06:18, 19 August 2024
chloride Calcium hydride Calcium oxide Calcium sulfate (Drierite) Cobalt(II) chloride Copper(II) sulfate Lithium chloride Lithium bromide Magnesium chloride...
1 KB (91 words) - 16:48, 2 October 2024
exchange. The compound is synthesized by the oxidation of a mixture of cobalt(II) chloride, lithium hydroxide, and ethylenediamine in the presence of carbon...
3 KB (240 words) - 20:11, 6 August 2024
electroplating. The compound can be prepared by treating nickel or nickel(II) carbonate with acetic acid: NiCO3 + 2 CH3CO2H + 3 H2O → Ni(CH3CO2)2·4 H2O + CO2...
6 KB (354 words) - 05:45, 21 September 2024
running off the piece. Colorants, such as iron oxide, copper carbonate or cobalt carbonate, and sometimes opacifiers including tin oxide and zirconium...
24 KB (2,863 words) - 06:02, 3 October 2024
Lithium-ion battery (redirect from Lithium-nickel-cobalt-aluminum)
level of the solution. Cobalt, the most expensive metal, can then be recovered in the form of sulfate, oxalate, hydroxide, or carbonate. [75] More recently...
208 KB (21,816 words) - 14:20, 25 October 2024
Blue pigments (section Cobalt blue)
8(AlSiO 4) 6(S,SO 4,Cl) 1–2. Cobalt pigments Cobalt blue (PB28): cobalt(II) aluminate. Cerulean blue (PB35): cobalt(II) stannate. Cerium uranium blue...
20 KB (2,218 words) - 20:14, 28 June 2024
substituted by nickel, cobalt or copper. Oxycarbonitrates containing an alkaline earth metal and cuprate and nitrate and carbonate anions in layers, form...
17 KB (1,055 words) - 01:49, 18 April 2024
copper carbonate (Cu2CO3(OH)2) is insoluble in water. True copper(II) carbonate (CuCO3) is rare and reacts with water to form basic copper carbonate. Anhydrous...
35 KB (1,083 words) - 11:43, 19 May 2024