In chemistry, a dihydrogen bond is a kind of hydrogen bond, an interaction between a metal hydride bond and an OH or NH group or other proton donor. With...
5 KB (592 words) - 21:05, 21 September 2024
assembly is bent. The hydrogen bond can be compared with the closely related dihydrogen bond, which is also an intermolecular bonding interaction involving hydrogen...
46 KB (5,463 words) - 19:48, 6 November 2024
minus one (as in dihydrogen, H2, with only one sigma bond, or ammonia, NH3, with 3 sigma bonds). There is no more than 1 sigma bond between any two atoms...
8 KB (918 words) - 13:02, 22 September 2024
Dihydrogen phosphate is an inorganic ion with the formula [H2PO4]−. Phosphates occur widely in natural systems. These sodium phosphates are artificially...
6 KB (486 words) - 21:39, 17 June 2024
a 1-electron bond is found in the dihydrogen cation, H+ 2. One-electron bonds often have about half the bond energy of a 2-electron bond, and are therefore...
28 KB (3,673 words) - 14:01, 24 October 2024
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force...
40 KB (4,872 words) - 13:33, 22 September 2024
example, the molecular orbital function for dihydrogen is an equal mixture of the covalent and ionic valence bond structures and so predicts incorrectly that...
12 KB (1,519 words) - 17:16, 5 November 2024
Molecular orbital diagram (category Chemical bonding)
1Σg+ for dihydrogen. Sometimes, the letter n is used to designate a non-bonding orbital. For a stable bond, the bond order defined as bond order = (...
39 KB (4,968 words) - 12:08, 22 September 2024
nitrate anion (NO−3), the bond order for each bond between nitrogen and oxygen is 4/3 (or 1.333333...). Bonding in dihydrogen cation H+2 can be described...
9 KB (1,260 words) - 05:21, 12 February 2023
of the bond between the hydrogen and deuterium in HD complexes. For example, JHD is 43.2 Hz in HD but 33.5 Hz in W(HD)(CO)3(PiPr3)2. Dihydrogen complexes...
7 KB (806 words) - 01:20, 5 October 2024
2-Norbornyl cation. Three-center four-electron bond 2-Norbornyl cation Dihydrogen complex I. Mayer (1989). "Bond orders in three-centre bonds: an analytical...
5 KB (688 words) - 12:00, 26 March 2023
The dihydrogen cation or hydrogen molecular ion is a cation (positive ion) with formula H+ 2. It consists of two hydrogen nuclei (protons), each sharing...
29 KB (3,536 words) - 20:31, 18 October 2024
Å, which can be compared with the H−H bonding distance of 0.74 Å. This interaction is called a dihydrogen bond. The original crystallographic analysis...
13 KB (1,032 words) - 07:30, 6 September 2024
orbitals. When creating the molecule dihydrogen, the individual valence orbitals, 1s, either: merge in phase to get bonding orbitals, where the electron density...
9 KB (1,093 words) - 22:34, 24 September 2022
capable of bonding with positively polarized hydrogen centres. This interaction, called dihydrogen bonding, is similar to hydrogen bonding, which exists...
21 KB (2,300 words) - 05:20, 19 November 2024
Properties of water (redirect from Dihydrogen oxide)
ionic compounds (which water is not). Water portal Chemical bonding of water Dihydrogen monoxide parody Double distilled water Electromagnetic absorption...
89 KB (9,582 words) - 13:48, 4 November 2024
10-Methacryloyloxydecyl dihydrogen phosphate (10-MDP, MDP Monomer) is a chemical compound used in dental adhesive materials. This organophosphate monomer...
8 KB (923 words) - 22:11, 31 October 2024
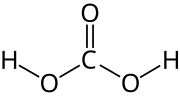
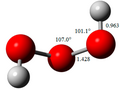
Carbonic acid (redirect from Dihydrogen carbonate)
(respectively 1.43 and 1.23 Å). The unusual C-O bond lengths are attributed to delocalized π bonding in the molecule's center and extraordinarily strong...
22 KB (2,178 words) - 00:41, 20 November 2024
Trioxidane (redirect from Dihydrogen trioxide)
Trioxidane (systematically named dihydrogen trioxide,), also called hydrogen trioxide is an inorganic compound with the chemical formula H[O] 3H (can be...
9 KB (903 words) - 21:55, 30 August 2024
Hydrogen (redirect from Dihydrogen)
is a gas of diatomic molecules with the formula H2, sometimes called dihydrogen, but more commonly called hydrogen gas, molecular hydrogen or simply hydrogen...
121 KB (12,377 words) - 14:27, 16 September 2024
Agostic interaction (redirect from Agostic bonding)
1016/s0065-3055(05)53006-5. ISBN 9780120311538. Kubas, G. J. (2001). Metal Dihydrogen and σ-Bond Complexes. New York: Kluwer Academic. ISBN 978-0-306-46465-2. Braga...
9 KB (925 words) - 06:24, 11 August 2024
tautomer with the non-classical dihydrogen complexes containing a M-(η2-H2) group. Borderline hydrides exist with a bond character somewhere between the...
6 KB (633 words) - 01:07, 18 August 2023
Hydrogen chalcogenide (redirect from Dihydrogen dichalcogenides)
Waals interactions, an effect of the large electron clouds of polonium. Dihydrogen dichalcogenides have the chemical formula H2X2, and are generally less...
20 KB (1,856 words) - 22:18, 15 April 2024
Disodium pyrophosphate is prepared by thermal condensation of sodium dihydrogen phosphate or by partial deprotonation of pyrophosphoric acid. Pyrophosphates...
12 KB (1,169 words) - 15:24, 13 November 2024
Ductility in Organic Plastic Crystals: Role of Molecular Shape and Dihydrogen Bonding Interactions in Aminoboranes". Angewandte Chemie International Edition...
9 KB (909 words) - 15:48, 10 November 2024
Trihydrogen cation (redirect from Protonated dihydrogen)
(A=3, N=0) (the common one). [DH2]+ = [2H1H2]+ (A=4, N=1) (deuterium dihydrogen cation). [D2H]+ = [2H21H]+ (A=5, N=2) (dideuterium hydrogen cation). D+3...
23 KB (2,741 words) - 07:33, 14 June 2024
Ester (redirect from Ester bond)
phosphate esters, e.g. triphenyl phosphate (O=P(−O−C6H5)3) and methyl dihydrogen phosphate (O=P(−O−CH3)(−OH)2) Pyrophosphoric (diphosphoric) acid forms...
46 KB (4,846 words) - 04:43, 20 November 2024
hydroborated and then the resulting vinylborane-based FLP can then activate dihydrogen. A protodeborylation step releases the cis-alkene product, which is obtained...
23 KB (2,669 words) - 14:31, 20 September 2024
Sulfuric acid (redirect from Dihydrogen sulfate)
above reaction is thermodynamically favored due to the high bond enthalpy of the Si–F bond in the side product. Protonation using simply fluoroantimonic...
64 KB (7,136 words) - 17:57, 1 November 2024
complex. Formation of a trigonal bipyramidal dihydrogen intermediate is followed by cleavage of the H–H bond, due to electron back donation into the H–H...
10 KB (1,210 words) - 14:28, 18 October 2024