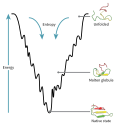The hydrophobic-polar protein folding model is a highly simplified model for examining protein folds in space. First proposed by Ken Dill in 1985, it is...
5 KB (647 words) - 23:24, 4 September 2023
The more positive the value, the more hydrophobic are the amino acids located in that region of the protein. These scales are commonly used to predict...
29 KB (3,414 words) - 17:32, 2 September 2024
protein folding, insertion of membrane proteins into the nonpolar lipid environment and protein-small molecule associations. Hence the hydrophobic effect...
13 KB (1,505 words) - 13:33, 21 August 2024
largely hydrophobic and can be visualized using the hydropathy plot. Depending on the number of transmembrane segments, transmembrane proteins can be classified...
21 KB (2,262 words) - 15:51, 18 July 2024
3D structure is the distribution of polar and non-polar side chains. Folding is driven by the burial of hydrophobic side chains into the interior of the...
71 KB (8,443 words) - 19:00, 15 August 2024
obtain, hydrophobic collapse is often studied in silico via molecular dynamics and Monte Carlo simulations of the folding process. Globular proteins that...
13 KB (1,429 words) - 20:28, 6 July 2023
can be tightly packed in the protein core in a hydrophobic environment, but they can also present at the polar protein surface. Each amino acid side...
71 KB (9,009 words) - 02:01, 27 September 2024
by the protein's tertiary structure. The molecule's apolar (hydrophobic) amino acids are bounded towards the molecule's interior whereas polar (hydrophilic)...
6 KB (746 words) - 14:10, 19 June 2024
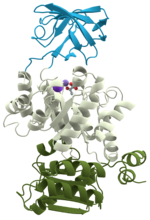
contributed an understanding of protein folding and structure mediated by hydrophobic interactions. The first protein to have its amino acid chain sequenced...
102 KB (11,361 words) - 18:20, 20 September 2024
proteins. The folding funnel hypothesis is closely related to the hydrophobic collapse hypothesis, under which the driving force for protein folding is...
19 KB (2,446 words) - 20:44, 26 September 2024
native state. This folding process is driven by the hydrophobic effect: a tendency for hydrophobic (water-fearing) portions of the protein to shield themselves...
24 KB (2,885 words) - 02:45, 28 March 2024
polybasic domain of the MARCKS protein or histactophilin, when their natural hydrophobic anchors are present. Lipid anchored proteins are covalently attached...
55 KB (4,391 words) - 21:04, 25 September 2024
and hydrophobic effects. Non-covalent interactions are critical in maintaining the three-dimensional structure of large molecules, such as proteins and...
28 KB (3,363 words) - 00:54, 15 December 2023
Denaturation (biochemistry) (redirect from Denatured protein)
environment. Protein folding consists of a balance between a substantial amount of weak intra-molecular interactions within a protein (Hydrophobic, electrostatic...
30 KB (3,178 words) - 21:52, 26 September 2024
similar energies, protein structures are dynamic, fluctuating between these similar structures. Globular proteins have a core of hydrophobic amino acid residues...
15 KB (1,603 words) - 16:54, 15 June 2024
the N-terminal. A span of 10 to 15 hydrophobic amino acids near the middle of the signal peptide. A slightly polar region near the C-terminal, typically...
52 KB (6,402 words) - 02:22, 14 August 2024
become known as the hydrophobic-polar model. It breaks the protein folding problem into three separate problems: modeling the protein conformation, defining...
16 KB (1,998 words) - 21:26, 25 September 2024
Ken A. Dill (category Molecular modelling)
introduced a toy model consisting of tethered beads on a lattice to mimic a folding protein, with beads of the same type (i.e. hydrophobic) attracting each...
4 KB (406 words) - 20:40, 16 February 2023
bi-layer: Integral proteins: Immersed in the bi-layer and held in place by the affinity of hydrophobic parts of the protein for the hydrophobic tails of phospholipids...
15 KB (1,994 words) - 06:34, 19 September 2024
sequences cannot sufficiently bury a hydrophobic core to fold into stable globular proteins. In some cases, hydrophobic clusters in disordered sequences provide...
52 KB (6,004 words) - 15:08, 6 May 2024
glycoprotein express different levels of protein activity. Polar cod (Boreogadus saida) exhibit similar protein activity and properties to the Antarctic...
52 KB (6,239 words) - 16:00, 3 September 2024
Beta sheet (redirect from Greek key (protein structure))
structural features present in whole proteins. Also proteins are inherently constrained by folding kinetics as well as folding thermodynamics, so one must always...
27 KB (3,120 words) - 01:54, 14 August 2024
Actin (redirect from Microfilament protein)
ensure that folding takes place correctly. CCT is a group II chaperonin, a large protein complex that assists in the folding of other proteins. CCT is formed...
153 KB (18,278 words) - 03:30, 28 September 2024
Cysteine (section Roles in protein structure)
in proteins. Therefore, cysteine is now often grouped among the hydrophobic amino acids, though it is sometimes also classified as slightly polar, or...
38 KB (3,795 words) - 22:20, 27 August 2024
of the major driving forces behind protein adsorption include: surface energy, intermolecular forces, hydrophobicity, and ionic or electrostatic interaction...
39 KB (4,870 words) - 08:34, 1 August 2024
Equilibrium unfolding (redirect from Protein thermodynamics)
the folded state (typically denoted N for "native" state) and the unfolded state (typically denoted U). This "all-or-none" model of protein folding was...
18 KB (3,111 words) - 05:35, 27 October 2021
termed inverse folding. Protein design is then an optimization problem: using some scoring criteria, an optimized sequence that will fold to the desired...
63 KB (7,659 words) - 10:45, 24 August 2024
forces, and hydrophobic effects. Macromolecular structure and function is dependent on the net effect of these forces (see protein folding), therefore...
5 KB (604 words) - 08:43, 17 September 2024
Beta barrel (category Protein folds)
the strands contain alternating polar and non-polar (hydrophilic and hydrophobic) amino acids, so that the hydrophobic residues are oriented into the interior...
15 KB (1,669 words) - 20:34, 23 March 2024
intramolecular interactions in the folded protein structure, including hydrogen bonding. Minimizing the number of hydrophobic side chains exposed to water by...
18 KB (2,345 words) - 15:28, 12 June 2024