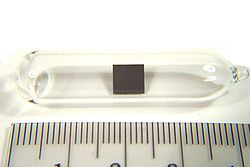
Niobium is a chemical element; it has symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and ductile transition...
85 KB (8,519 words) - 23:49, 7 March 2025
Plutonium (section Isotopes and nucleosynthesis)
to power some spacecraft. Plutonium isotopes are expensive and inconvenient to separate, so particular isotopes are usually manufactured in specialized...
140 KB (15,133 words) - 08:42, 23 February 2025
Praseodymium (section Isotopes)
isotopes lighter than 141Pr is positron emission or electron capture to isotopes of cerium, while that of heavier isotopes is beta decay to isotopes of...
38 KB (5,021 words) - 18:09, 8 April 2025