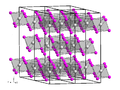
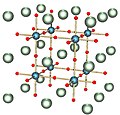
Lutetium(III) iodide or lutetium iodide is an inorganic compound consisting of iodine and lutetium, with the chemical formula of LuI3. Lutetium(III) iodide...
4 KB (359 words) - 02:59, 8 September 2023
solutions of most lutetium salts are colorless and form white crystalline solids upon drying, with the common exception of the iodide. The soluble salts...
16 KB (1,801 words) - 11:58, 24 May 2024
Scandium triiodide (redirect from Scandium(III) iodide)
triiodide, also known as scandium iodide, is an inorganic compound with the formula ScI3 and is classified as a lanthanide iodide. This salt is a yellowish powder...
3 KB (236 words) - 04:48, 1 January 2022
Iodine (section Hydrogen iodide)
example, molybdenum(IV) oxide reacts with aluminium(III) iodide at 230 °C to give molybdenum(II) iodide. An example involving halogen exchange is given below...
104 KB (11,657 words) - 18:48, 27 October 2024
Ytterbium (section Yb(II) vs. Yb(III))
"lutecia" was separated from ytterbia, from which the element "lutecium" (now lutetium) was extracted by Georges Urbain, Carl Auer von Welsbach, and Charles James...
41 KB (5,222 words) - 10:29, 13 August 2024
fluoride − AmF3 Americium(IV) fluoride − AmF4 Americium(II) iodide − AmI2 Americium(III) iodide − AmI3 Americium dioxide – AmO2 Ammonia – NH3 Ammonium azide...
119 KB (8,735 words) - 17:31, 25 October 2024
Thallium (section Thallium(III))
thallium doping is the sodium iodide and cesium iodide crystals in gamma radiation detection devices. In these, the sodium iodide crystals are doped with a...
52 KB (5,787 words) - 22:55, 29 September 2024
fluoride, sodium chloride (common table salt), silver bromide and potassium iodide. The group of halogens is the only periodic table group that contains elements...
52 KB (5,499 words) - 18:00, 11 September 2024
lutetium iodide 13813–45–1 LuN lutetium nitride 12125–25–6 Lu(NO3)3 lutetium nitrate 36549–50–5 Lu2O3 lutetium oxide 12032–20–1 Lu2S3 lutetium sulfide...
139 KB (120 words) - 17:07, 15 July 2024
uranium(III) chloride (space group P63/m) and the melting point of 715 °C. The fluoride is isotypic to LaF3 (space group P63/mmc) and the iodide to BiI3...
77 KB (9,268 words) - 15:13, 11 October 2024
Copper (redirect from Copper(III))
fluorine, chlorine, and bromine. Attempts to prepare copper(II) iodide yield only copper(I) iodide and iodine. 2 Cu2+ + 4 I− → 2 CuI + I2 Copper forms coordination...
122 KB (13,910 words) - 18:45, 24 October 2024
Isotopes of hafnium and lutetium (along with ytterbium) are also used in isotope geochemistry and geochronological applications, in lutetium-hafnium dating. It...
47 KB (5,420 words) - 01:54, 24 October 2024
mineral acids are rapid. Californium is only water-soluble as the californium(III) cation. Attempts to reduce or oxidize the +3 ion in solution have failed...
44 KB (4,631 words) - 23:46, 21 August 2024
is +3 for all halides from fluoride to iodide. Einsteinium(III) fluoride (EsF3) can be precipitated from Es(III) chloride solutions upon reaction with...
67 KB (7,944 words) - 07:00, 24 October 2024
electrolysis at 12.3% efficiency use perovskite photovoltaics. Cerium-doped lutetium aluminum perovskite (LuAP:Ce) single crystals were reported. The main property...
48 KB (4,994 words) - 15:00, 10 September 2024
Uranium (redirect from Uranium(V) iodide)
chlorine. All uranium chlorides react with water and air. Bromides and iodides of uranium are formed by direct reaction of, respectively, bromine and...
110 KB (12,380 words) - 11:06, 17 October 2024
fluoride, chloride, and bromide have the sodium chloride structure, but the iodide has three known stable forms at different temperatures; that at room temperature...
94 KB (11,264 words) - 05:57, 19 October 2024
Praseodymium(III) iodide can be prepared by heating praseodymium and iodine in an inert atmosphere produces praseodymium(III) iodide, or by heating...
17 KB (1,694 words) - 15:23, 17 April 2024
C.P. (1962). "Preparation of anhydrous lanthanide halides, especially iodides". Journal of Inorganic and Nuclear Chemistry. 24 (4): 387–391. doi:10...
7 KB (406 words) - 15:23, 12 July 2024
Indium (section Indium(III))
alkalis. Indium(I) compounds are not common. The chloride, bromide, and iodide are deeply colored, unlike the parent trihalides from which they are prepared...
49 KB (5,783 words) - 18:47, 26 October 2024
Lanthanide (section Ln(III) compounds)
lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (element 71) is also sometimes considered a lanthanide, despite being a...
107 KB (10,324 words) - 11:27, 28 October 2024
tetrabenzylhafnium. Hafnium does form lower halides such as hafnium(III) iodide. Hafnium trihalides are strongly reducing compounds and as such do not...
15 KB (1,823 words) - 05:55, 30 April 2024
Scandium fluoride (redirect from Scandium(III) flouride)
Scandium(III) fluoride, ScF3, is an ionic compound. This salt is slightly soluble in water but dissolves in the presence of excess fluoride to form the...
7 KB (612 words) - 07:46, 16 October 2024
ytterbium(III) chloride (YbCl3) is a Lewis acid and can be used as a catalyst in the Aldol and Diels–Alder reactions. Ytterbium(II) iodide (YbI2) may...
16 KB (1,523 words) - 15:35, 17 April 2024
Ellis BD, MacDonald CL (2004). "Stabilized Arsenic(I) Iodide: A Ready Source of Arsenic Iodide Fragments and a Useful Reagent for the Generation of Clusters"...
130 KB (14,331 words) - 18:11, 27 October 2024
Bierbach, Wolfgang Saak; "FeI3SC(NMe2)2, a Neutral Thiourea Complex of Iron(III) Iodide", Angewandte Chemie International Edition in English (1989) 28 (6), 776-777...
150 KB (17,078 words) - 12:22, 24 August 2024
zincblende (cubic) or wurtzite (hexagonal) form. This group is also known as the III-V family of compounds. The Heusler structure, based on the structure of Cu2MnAl...
49 KB (3,620 words) - 14:56, 4 October 2024
Aluminium fluoride (redirect from Aluminum(III) fluoride)
Scandium(III) fluoride Yttrium(III) fluoride Lutetium(III) fluoride Lanthanum(III) fluoride Cerium(III) fluoride Actinium(III) fluoride Except where otherwise...
14 KB (1,283 words) - 09:37, 11 April 2024
deficit then appears for holmium through lutetium with the average coordination number dropping to 8.2 at lutetium(III). The configuration is maintained despite...
71 KB (7,653 words) - 21:25, 1 May 2024
holmium(III) bromide and holmium(III) iodide, can be obtained by the direct reaction of the elements: 2 Ho + 3 X2 → 2 HoX3 In addition, holmium(III) iodide can...
37 KB (4,312 words) - 13:41, 23 October 2024