polyproline helix is a type of protein secondary structure which occurs in proteins comprising repeating proline residues. A left-handed polyproline II...
10 KB (1,313 words) - 13:58, 24 July 2024
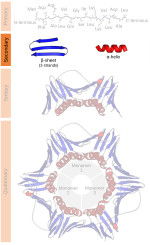
unfavorable backbone packing in the center of the helix. Other extended structures such as the polyproline helix and alpha sheet are rare in native state proteins...
28 KB (3,076 words) - 03:33, 27 October 2024
chain. Multiple prolines and/or hydroxyprolines in a row can create a polyproline helix, the predominant secondary structure in collagen. The hydroxylation...
23 KB (2,241 words) - 00:20, 13 October 2024
helix is made of three collagen peptides, each of which forms its own left-handed polyproline helix. When the three chains combine, the triple helix adopts...
12 KB (1,553 words) - 19:12, 4 August 2024
FASCICULIN-II 1vzj: STRUCTURE OF THE TETRAMERIZATION DOMAIN OF ACETYLCHOLINESTERASE: FOUR-FOLD INTERACTION OF A WWW MOTIF WITH A LEFT-HANDED POLYPROLINE HELIX...
35 KB (3,880 words) - 16:55, 22 July 2024
dimeric transmembrane β-helix. This peptide is secreted by gram-positive bacteria as an antibiotic. A transmembrane polyproline-II helix has not been reported...
21 KB (2,262 words) - 15:51, 18 July 2024
structure exon 1 and exon 3 corresponding peptides may take on is the polyproline helix (PPII), indicated by the high occurrence of proline and glycine in...
25 KB (3,122 words) - 13:35, 14 January 2024
his co-worker Vladimir Kubyshkin, the new-to-nature hydrophobic polyproline-II helix foldamer was designed. Along with Budisa's previous work on bioexpression...
23 KB (2,131 words) - 04:55, 9 November 2024
the polypeptide has the conformation of beta sheet or of type II polyproline helix (PPII). A number of glutamines and asparagines help bind short peptides...
3 KB (409 words) - 04:38, 26 April 2024
interesting secondary structure as a cluster of proline can form a polyproline helix. PRR12 contains a possible nuclear import signal starting at P1794...
12 KB (1,301 words) - 14:54, 11 April 2024
most common regions are labeled: α for α helix, Lα for left-handed helix, β for β-sheet, and ppII for polyproline II. Such a clustering is alternatively...
16 KB (1,761 words) - 05:00, 29 October 2024
located from P185 to P188 has the secondary structure of a type II polyproline helix. This gene is found in primates but is also found at a very poor E-values...
10 KB (1,162 words) - 22:51, 2 December 2023
C-α-branched side chains are known to adopt structure analogous to polyproline I helix. Different strategies have been employed to predict and characterize...
17 KB (1,907 words) - 14:02, 2 August 2023
PPI may refer to: PPi, the anion P2O74−, a pyrophosphate Polyproline I helix Protein–protein interaction Patient and public involvement Prepulse inhibition...
2 KB (236 words) - 12:24, 26 February 2024
domains. Binding of the enzyme to the thylakoid membrane involves a polyproline type II helix created between two FNR monomers and several proline rich integral...
18 KB (1,997 words) - 19:12, 27 March 2024
acids are bound together to form a triple helix of elongated fibril known as a collagen helix. The collagen helix is mostly found in connective tissue such...
69 KB (7,689 words) - 16:34, 6 November 2024
share the defining structural feature known as the triple helix, where three left handed polyproline II-type (PPII) helices assemble to form a right-handed...
18 KB (1,928 words) - 17:41, 18 April 2024
ligands. A subset of WH1 domains has been termed the EVH1 domain bind a polyproline motif. The EVH1 domain (also known as the WH1, RanBP1-WASP, or enabled/VASP...
8 KB (908 words) - 14:47, 19 October 2024
short genes containing the characteristic collagen-triple helix sequence, as well as polyproline domains and cystein-rich domains. Trimers of minicollagen...
24 KB (2,866 words) - 23:33, 30 September 2024
research on synthetic peptides derived from abductin were found to have polyproline II helix structure in aqueous solutions and type II β-turn structure in hydrophobic...
16 KB (2,142 words) - 04:49, 25 July 2023
receptor by Pro-Leu-Gly-NH2 peptidomimetics constrained in either a polyproline II helix or a type II beta-turn conformation". Journal of Medicinal Chemistry...
17 KB (1,892 words) - 23:03, 6 August 2024
"The synaptic acetylcholinesterase tetramer assembles around a polyproline II helix". EMBO J. 23 (22): 4394–405. doi:10.1038/sj.emboj.7600425. PMC 526459...
9 KB (836 words) - 21:59, 13 June 2024
N-substituted polyglycines that utilize steric interactions to fold into polyproline type-I-like helical structures. Aedamers that fold in aqueous solutions...
15 KB (1,668 words) - 23:25, 15 March 2024
2020-11-03. Adzhubei AA, Sternberg MJ, Makarov AA (June 2013). "Polyproline-II Helix in Proteins: Structure and Function". Journal of Molecular Biology...
30 KB (3,569 words) - 10:35, 25 July 2024
carbohydrate response element sequences of DNA. This gene encodes a basic helix-loop-helix leucine zipper transcription factor of the Myc / Max / Mad superfamily...
8 KB (891 words) - 16:48, 9 May 2024
This highly conserved N-terminal domain mediates NEDD9 binding to the polyproline motifs of a number of important interacting proteins, with some well-studied...
64 KB (8,012 words) - 11:54, 5 January 2024
helices, with each leucine residue positioned on one face of the alpha helix to form a hydrophobic protein-binding interface. The N-terminal region also...
25 KB (3,036 words) - 15:57, 21 December 2023
any amino acid. Each 3-amino-acid repeat forms one turn of a polyproline type II helix. The helices then fold together, to form a bundle that is two...
52 KB (6,271 words) - 20:14, 2 November 2024
with other synthetic RNAs, they found that poly-C directed synthesis of polyproline. Nirenberg recounts that the labs of Severo Ochoa and James Watson had...
15 KB (1,913 words) - 03:00, 5 June 2024
monomers are soluble and contain short regions of beta sheet and polyproline II helix secondary structures in solution, though they are largely alpha helical...
65 KB (7,761 words) - 09:06, 10 September 2024